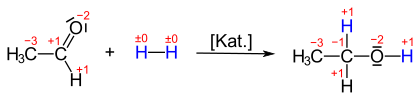Reduction (chemistry)
A reduction is a chemical reaction in which one or more electrons are absorbed by a particle ( atom , ion or molecule ). This lowers the oxidation number of the constituent particle that is reduced by the number of electrons accepted. A reduction always occurs together with the oxidation of the particle that supplied the electrons, which is known as the reducing agent . In the reducing agent, the oxidation number of the constituent particle that has supplied the electrons is increased by the number of the supplied electrons. Both coupled reactions are known as the redox reaction .
history
In the early days of chemistry, reduction was viewed as the removal of oxygen from an oxide . A reaction in which an oxidation was reversed was called reduction (from the Latin reductio for "return") . Oxidation was defined as the union of a compound or an element with oxygen, which was based on the findings of Antoine Laurent de Lavoisier .
Oxides of noble metals, such as silver (I) oxide , decompose when they are simply heated. Oxygen and elemental silver are formed from silver (I) oxide .
If copper (II) oxide is heated in a hydrogen stream, metallic copper and water are produced . Hydrogen acts here as a reducing agent and removes oxygen from the copper (II) oxide.
Today, a broader perspective applies, which is not limited to reactions of oxygen-containing compounds and has integrated the classic perspective.
general definition
Reduction is a reaction in which a mono- or polyatomic particle Ox accepts one or more electrons. The particle Red is formed:
Ox reacts as an electron acceptor , Ox and Red form a so-called redox couple . The electrons come from a second redox pair that is subject to oxidation. While in the field of electrochemistry , as in electrolysis or a galvanic cell , the electron transfer between the two redox pairs is a measurable variable, in other cases the reduction can only be recognized by the associated lowering of the oxidation number of Ox.
If a reduction is viewed as an equilibrium reaction, the reverse reaction is an oxidation. Such equilibria exist, for example, in an unused accumulator . While the partial reaction runs in one direction during discharging, charging leads to a reversal of the reaction direction.
Although a reduction never occurs without oxidation and therefore a redox reaction occurs, a reaction is often viewed from the perspective of the desired product. We speak of a reduction of iron ore to elemental iron or a cathodic reduction of aluminum oxide to aluminum .
Absorption of electrons - reduction of the oxidation number
If an iron nail is placed in an aqueous copper (II) sulfate solution , a reddish-brown coating of metallic copper forms on the nail. The copper is reduced and the iron oxidizes to Fe 2+ ions.
The iron, which is itself oxidized during the redox reaction, is also called reducing agent in this context , because its presence enables the copper to be reduced. Reduction always means a decrease in the oxidation number due to the uptake of electrons. Oxidation, on the other hand, means the release of electrons and thus an increase in the oxidation number. In this case, the charges on the particles correspond to their oxidation number.
The thermal decomposition of silver (I) oxide with the oxidation states is also a reaction in which electrons are transferred.
When copper oxide is reacted with hydrogen, copper is reduced. Hydrogen acts as a reducing agent here. The oxidation state of the oxygen atoms remains unchanged in the reaction, but the atoms change their binding partner. The formally formed H + ions combine with the formally unchanged O 2− ions to form the reaction product water with the oxidation states .
Addition of hydrogen
An uptake of hydrogen by organic compounds leads to a reduction in the oxidation number of one or more carbon atoms. The catalytic hydrogenation of 2-butene leads to n -butane :
The oxidation numbers of the carbon atoms formerly linked by double bonds change from −1 to −2. Formally, these atoms each have one electron and one proton . The 2nd redox pair with the reducing agent H 2 can be formulated as follows:
In this reaction, hydrogen is formally oxidized and the electrons are formally released. The overall reaction is a redox reaction; from the perspective of the educt 2-butene, the compound is reduced to n- butane. Often this reaction is viewed as an addition reaction . From this point of view, the changes in oxidation states are irrelevant.
Reduction is often used in connection with oxygen-containing organic compounds, such as the conversion of ketones or aldehydes into alcohols . If acetaldehyde absorbs hydrogen, ethanol is produced . The oxidation state of the carbonyl group changes from +1 to −1:
Reductions are important in biochemistry . In many metabolic pathways in a cell , a reduction takes place through the transfer of hydrogen. Coenzymes such as NADH , NADPH or FADH are capable of formally transferring a hydride ion or hydrogen to another compound.
See also
Individual evidence
- ↑ Entry on reduction . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.R05222 Version: 2.3.1.
- ↑ Duden , German Universal Dictionary , 4th ed., Mannheim, 2001; see also Duden online
- ↑ PONS Online German - Latin
- ^ Arnold F. Holleman, Nils Wiberg: Textbook of Inorganic Chemistry , 102nd Edition, de Gruyter, Berlin, 2007, p. 218ff.
- ↑ Paula Yurkanis Bruice: Organic Chemistry , Pearson Education, Munich, 5th edition, 2011, p 823ff.
- ↑ Reverse reaction according to the 3rd criterion of the definition of an oxidation, see entry on oxidation . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.O04362 Version: 2.3.1.
![\ mathrm {2 \ Ag_2O \ \ xrightarrow [] {\ Delta} \ 4 \ Ag \ + \ O_2}](https://wikimedia.org/api/rest_v1/media/math/render/svg/92b1a2d90a7393866541de09f446b2b0678982bd)
![\ mathrm {CuO \ + \ H_2 \ \ xrightarrow [] {\ Delta} \ Cu \ + \ H_2O}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f6080949767d7c06542708d1222f2e9f2b46fa78)


![\ mathrm {\ \ xrightarrow [] {reduction}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6cbe51c4c320c6601a67b9a9bcd8d76d527245ba)

![\ mathrm {\ \ xleftarrow [oxidation] {}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4048ca773f2df6398d9db2dfb0ff737cff823884)