Copper (II) oxide
| Crystal structure | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
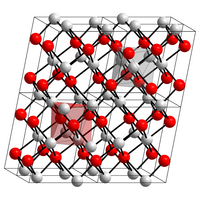
|
||||||||||||||||||||||
| __ Cu 2+ __ O 2− | ||||||||||||||||||||||
| Crystal system |
monoclinic |
|||||||||||||||||||||
| Space group |
C 2 / c (No. 15) |
|||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Copper (II) oxide | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Ratio formula | CuO | |||||||||||||||||||||
| Brief description |
black, amorphous or crystalline solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 79.545 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
6.48 g cm −3 |
|||||||||||||||||||||
| Melting point |
1326 ° C |
|||||||||||||||||||||
| boiling point |
thermal decomposition: 1026 ° C |
|||||||||||||||||||||
| solubility |
almost insoluble in water, soluble in dilute acids, soluble in ammonium hydroxide |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
0.1 mg m −3 (measured as the inhalable aerosol fraction) |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Copper (II) oxide (formerly known as copper ash , copper rust , burnt copper and copper hammer blow or copper blow) is a chemical compound that contains copper and oxygen . In this oxide with the empirical formula CuO, copper is divalent . Copper (II) oxide is a black, amorphous or crystalline solid.
Occurrence
Copper (II) oxide occurs naturally as the mineral tenorite . Tenorite is formed during the weathering of copper sulfides and is therefore usually found in oxidized parts of copper deposits. The mineral is named after the Italian botanist Michele Tenore .
Extraction and presentation
Copper (II) oxide can be produced by thermal decomposition ( pyrolysis ) of copper (II) nitrate or basic copper carbonate .
Copper (II) oxide can also be produced by heating (freshly precipitated) copper (II) hydroxide . The copper (II) hydroxide is precipitated by adding alkali metal hydroxides to a Cu (II) salt solution.
Copper (II) oxide forms together with copper (I) oxide when metallic copper is heated to red heat in air.
properties
Copper (II) oxide is insoluble in water and alcohols . In contrast, it is soluble in dilute acids . The corresponding copper (II) salts can be obtained by evaporation. Copper (II) oxide is soluble in ammonia water with complex formation .
Copper (II) oxide easily adsorbs oxygen, nitrogen , carbon dioxide and other gases .
When heated above about 800 ° C, copper (II) oxide is reduced to copper (I) oxide with the release of oxygen.
If metallic copper and copper (II) oxide are heated together , copper (I) oxide is also formed .
Copper (II) oxide is reduced to metallic copper by various reducing agents (e.g. carbon , carbon monoxide , hydrogen ) at elevated temperatures .
When copper (II) oxide is heated with hydrogen fluoride to 400 ° C, copper (II) fluoride is formed.
It has a monoclinic crystal structure with the space group C 2 / c (space group no. 15) (a = 4.683 Å , b = 3.423 Å, c = 5.129 Å, β = 95.54 °). Its enthalpy of formation is −155.8 kJ / mol.
use
Copper (II) oxide is used as a pigment for coloring glass , ceramics , porcelain and artificial gemstones . In addition, it is used as a cathode material in batteries , as a catalyst , for desulfurization of crude oil and antifouling paints. Copper (II) oxide is also used as a raw material for the production of various copper compounds. Since the discovery of the superconductivity of compounds of La 2 CuO 4 (doped with strontium) and the subsequent discovery of more than a hundred similar compounds, most of which cannot do without copper and oxygen, copper (II) oxide is also used for ceramic Superconductors are used, which are considered future-oriented materials.
Individual evidence
- ↑ a b c d e f g Entry on copper (II) oxide in the GESTIS substance database of the IFA , accessed on December 7, 2019(JavaScript required) .
- ↑ Entry on copper oxides. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ Entry on copper (II) oxide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on June 17, 2017. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ Emil Ernst Ploß: A book of old colors. Technology of textile colors in the Middle Ages with an outlook on solid colors. 6th edition Munich 1989, ISBN 978-3-89164-060-9 .
- ^ Wolfgang Schneider: Pharmaceutical chemicals and minerals. Supplements (to Volume III of the Lexicon for the History of Medicines). Frankfurt am Main 1975, p. 85.
- ↑ Mindat: Tenorite (Eng.)
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 979.











