Copper (II) selenide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
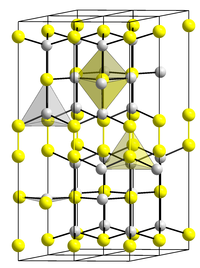
|
||||||||||||||||
| __ Cu 2+ __ Se 2− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Copper (II) selenide | |||||||||||||||
| other names |
Copper selenide (ambiguous) |
|||||||||||||||
| Ratio formula | CuSe | |||||||||||||||
| Brief description |
black solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 142.50 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
6.0-6.6 g cm -3 |
|||||||||||||||
| Melting point |
550 ° C (decomposition) |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Copper (II) selenide is an inorganic chemical compound of copper from the group of selenides .
Occurrence
Copper (II) selenide occurs naturally in the form of the mineral klockmannite .
Extraction and presentation
Copper (II) selenide can be obtained by peritectic decomposition of copper diselenide CuSe 2 ( Kruťait ).
properties
Copper (II) selenide is a black solid that is insoluble in water. It is soluble in hydrogen chloride with evolution of selenium and soluble in sulfuric acid with evolution of sulfur dioxide . It is oxidized to copper (II) selenite CuSeO 3 by nitric acid. Copper (II) selenide occurs in three modifications. The α-form present at room temperature has a hexagonal crystal structure of the klockmannite type with the space group P 6 3 / mmc (space group no. 194) and is isotypic to that of copper (II) sulfide , the β present from 51 ° C -Form has an orthorhombic crystal structure and the γ-form present from 157 ° C again has a hexagonal crystal structure with the space group P 6 3 / mmc (No. 194) .
use
Copper (II) selenide is used as a semiconductor and as a catalyst in Kjeldah digestion .
Individual evidence
- ↑ a b c d e f g h i data sheet Copper (II) selenide, 99.5% (metals basis) from AlfaAesar, accessed on April 11, 2014 ( PDF )(JavaScript required) .
- ↑ a b D. J. Chakrabarti, DE Laughlin: The Cu / Se (Copper-Selenium) system. In: Bulletin of Alloy Phase Diagrams. 2, 1981, pp. 305-315, doi : 10.1007 / BF02868284 .
- ^ Helmut Schrätze, Karl-Ludwig Weiner: Mineralogie: A textbook on a systematic basis - Helmut Schrätze, Karl-Ludwig Weiner . Walter de Gruyter, 1981, ISBN 3-11-083686-6 , p. 223 ( limited preview in Google Book search).


