Gold (I) sulfide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ Au + __ S 2− | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Gold (I) sulfide | ||||||||||||||||||
| other names |
Digold sulfide |
||||||||||||||||||
| Ratio formula | Au 2 S | ||||||||||||||||||
| Brief description |
brown-black solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 426.00 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
11.0 g cm −3 |
||||||||||||||||||
| Melting point |
Decomposition from 217 ° C |
||||||||||||||||||
| solubility |
almost insoluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Gold (I) sulfide into a chemical compound of gold and sulfur . In addition to gold (III) sulfide, the black-brown solid is one of the two well-known gold sulfides .
In addition to the solid, it is also possible to synthesize nanoparticles , which have different optical and electronic properties compared to the solid.
Extraction and presentation
Gold (I) sulfide can be obtained from gold cyanide solutions with the aid of hydrogen sulfide . For this purpose, gold is first dissolved in a potassium cyanide solution with access to oxygen . The sulphide can then be precipitated out of the solution with hydrogen sulphide.
-
- Loosening the gold
-
- Precipitation of gold (I) sulfide
properties
Physical Properties
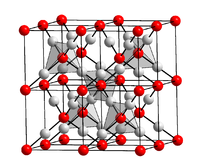
Gold (I) sulfide crystallizes in the same crystal structure as copper (I) oxide . The structure is cubic with the space group Pn 3 m (space group no. 224) and the lattice parameter a = 502 pm .
Between 25 and 100 ° C, gold (I) sulfide is a p-type semiconductor with a band gap of 0.37 eV .
Chemical properties
Gold (I) sulfide decomposes from 217 ° C. In addition to elemental gold, sulfur dioxide is produced in the air .
Gold (I) sulfide is insoluble in water, but it decomposes into gold and hydrogen sulfide on contact with acids.
Retinal diseases caused by gold sulfides
Poisoning with gold compounds is extremely rare; only when dealing with soluble gold salts such as potassium dicyanoaurate (I) are among workers in electroplating companies in addition to allergic reactions which also by deposits of gold sulfides diseases caused retina ( retina ) of the eye (retinopathy) occurred.
Individual evidence
- ↑ a b gold (I) sulfide at webelements.com
- ↑ a b c d e K. Ishikawa, T. Isonaga, S. Wakita, Y. Suzuki: Structure and electrical properties of Au 2 S . In: Solid State Ionics , 1995, 79, pp. 60-66, doi : 10.1016 / 0167-2738 (95) 00030-A .
- ^ A b c Todd Morris, Hollie Copeland, Greg Szulczewski: Synthesis and Characterization of Gold Sulfide Nanoparticles . In: Langmuir , 2002, 18 2, pp. 535-539, doi : 10.1021 / la011186y .
- ↑ a b data sheet gold (I) sulfide from Sigma-Aldrich , accessed on April 3, 2011 ( PDF ).
- ^ E. Burgis: Intensive course: General and special pharmacology. Elsevier Germany, 2005, ISBN 978-3-437-42612-4

![{\ displaystyle \ mathrm {4 \ Au + 8 \ KCN + 2 \ H_ {2} O + O_ {2} \ longrightarrow 4 \ K [Au (CN) _ {2}] + 4 \ KOH}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/24512a58c1a8619a4384892ca5f8d288b9804a4f)
![{\ displaystyle \ mathrm {2 \ K [Au (CN) _ {2}] + H_ {2} S + 2 \ H_ {2} O \ longrightarrow Au_ {2} S + 2 \ KOH + 4 \ HCN}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/70ec0bd0c0897ec7cebdba7b576d992ad0c764dc)