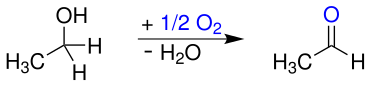acetaldehyde
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | acetaldehyde | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 2 H 4 O | ||||||||||||||||||
| Brief description |
colorless liquid or gas with a pungent odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 44.1 g mol −1 | ||||||||||||||||||
| Physical state |
liquid or gaseous |
||||||||||||||||||
| density |
|
||||||||||||||||||
| Melting point |
−123 ° C |
||||||||||||||||||
| boiling point |
20 ° C |
||||||||||||||||||
| Vapor pressure |
1006 h Pa (20 ° C) |
||||||||||||||||||
| pK s value |
13.57 (25 ° C) |
||||||||||||||||||
| solubility |
miscible with water |
||||||||||||||||||
| Dipole moment | |||||||||||||||||||
| Refractive index |
1.3316 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
|
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Acetaldehyde [ aˈt͡seːt | aldehyːt ], also called ethanal , is an aldehyde and has the semi-structural formula CH 3 -CHO.
In the human body, acetaldehyde is formed as an intermediate product when ethanol is broken down by alcohol dehydrogenase . Acetaldehyde, among other substances, is responsible for the " hangover " the next morning. Acetaldehyde is usually quickly metabolized to acetate . After taking disulfiram or coprin (active ingredient in the wrinkle ink ), the metabolism of acetaldehyde is inhibited and it accumulates in the body, which is associated with (mostly mild) symptoms of intoxication ( antabus or coprinus syndrome ). When tobacco is smoldered or burned, it arises as a by-product / pyrolysis product and thus enters the blood from the tobacco smoke via the alveoli. In addition, acetaldehyde was found in plant extracts, essential oils , roasted coffee and mineral water (especially in the own brands of various discounters packed in plastic ). Some deciduous trees, such as B. maple and poplar , give off acetaldehyde when changing from light to dark.
From a biochemical point of view, acetaldehyde is also a common intermediate. To transform yeast the in glycolysis resulting pyruvate in two steps to ethanol to by the pyruvate first using the pyruvate decarboxylase, an enzyme converted the class of the lyases, in acetaldehyde and then with the aid of alcohol dehydrogenase , an enzyme of the class of oxidoreductases , is converted into ethanol.
nomenclature
The IUPAC systematic name ethanal is derived from ethane by adding the suffix -al for aldehydes . The commonly preferred name acetaldehyde goes back to “acetum”, the Latin word for vinegar , because the oxidation of acetaldehyde (“acetic acid aldehyde”) produces acetic acid .
history
Acetaldehyde was probably first synthesized in 1781 by Carl Wilhelm Scheele while attempting to oxidize ethanol with manganese dioxide in the presence of sulfuric acid. The characterization was only achieved by Justus Liebig in collaboration with Johann Wolfgang Döbereiner in 1835. He named the compound aldehyde (from Latin al coholus dehyd rogenatus). Acetaldehyde can thus be regarded as the historically first representative of the class of aldehydes, while formaldehyde (methanal) is the simplest representative of the class.
Extraction and presentation
Historically acetaldehyde by oxidation of ethanol with a number of oxidizing agents such as manganese dioxide / sulfuric acid (Scheele) or chromic acid obtained (Liebig), according to the following equation:
For large-scale synthesis , acetaldehyde is produced in the Wacker-Hoechst process by catalytic addition of water to ethene with simultaneous air oxidation using fixed-bed catalysts. Formally, the following equation results:
In the 1970s, the global production capacity for acetaldehyde rose to over 2 million tons per year. However, due to the development of new synthetic routes that do not require acetaldehyde as a starting material , the demand is currently falling.
properties
Acetaldehyde is a colorless, very volatile and easily flammable liquid that can be mixed with water in any proportion; the aldehyde hydrate is formed in an equilibrium reaction . In contrast to formaldehyde, however , the equilibrium is only slightly over 50% on the hydrate side.
Acetaldehyde must be stored in a cool place, as it boils at 20 ° C and forms explosive vapor-air mixtures. Due to the extremely low ignition point of 140 ° C, these vapors can ignite on hot heating surfaces. The flash point of acetaldehyde is -39 ° C and its UN number is 1089.
Acetaldehyde oligomerizes easily with acid catalysis to form aldol addition products.
- The “dimer” (CH 3 CHO) 2 is the so-called aldol .
- The trimer (CH 3 CHO) 3 has a cyclic acetal structure (2,4,6-trimethyl-1,3,5-trioxane). It is a liquid (bp. 124 ° C) with the common name paraldehyde .
- The tetramer (CH 3 CHO) 4 also has a cyclic acetal structure (2,4,6,8-tetramethyl-1,3,5,7-tetroxocane). It is a solid (sublimed at 112 ° C), also called metaldehyde . It is used as a dry fuel and, because of its toxicity, also as snail poison ( slug pellets ).
- In most cases, dry fuel also contains higher oligomers of acetaldehyde, such as. B. Pentamers (CH 3 CHO) 5 and Hexamers (CH 3 CHO) 6 .
The oligomers with an acetal structure can easily be cleaved again by acids.
Tautomerism
Acetaldehyde has an unstable tautomer , vinyl alcohol or ethenol . This simplest enol isomerizes immediately to acetaldehyde in the free state under normal conditions, provided it is not stabilized - for example as an iron carbonyl or platinum complex Pt (acac) (η 2 -C 2 H 3 OH) Cl.
Effects in the human body
Acetaldehyde easily bonds with human DNA and is therefore mutagenic (mutagenic) and also carcinogenic (carcinogenic). First, acetaldehyde can undergo a reversible reaction with nucleosides in DNA. The resulting unstable adducts can be converted into stable adducts by reduction with sodium borohydride . After such a reduction, a guanosine-acetaldehyde adduct is converted into a product to which the structure of N2-ethylguanosine is ascribed.
Acetaldehyde has a number of harmful effects on the liver and heart . It forms protein adducts that activate the so-called Kupffer cells ( macrophages of the liver). These increasingly secrete substances that change other cells of the liver, the Ito cells, in such a way that they then produce more collagen . This favors the development of liver cirrhosis . In addition, by activating NADPH oxidase (NOX2), acetaldehyde leads to the increased formation of oxygen radicals , which damage the membranes of the cells so that they perish. This also affects the mitochondria of the cardiomyocytes , which initially affects the ability of the heart muscle cells to contract and then destroys them, resulting in irreparable damage to the muscle and ultimately chronic heart failure .
use
Acetaldehyde is an important raw material in the chemical industry. Acetaldehyde is used, for example, to produce acetic acid , acetic anhydride , butadiene , acrolein and pentaerythritol .
Web links
- www.wissenschaft.de: What hangovers and cancer have in common - the alcohol breakdown product acetaldehyde causes a heavy head and increases the risk of cancer in the gastrointestinal tract
- Deutschlandfunk: Change in the assessment of acetaldehyde
Individual evidence
- ↑ a b c d e f g Entry on acetaldehyde in the GESTIS substance database of the IFA , accessed on November 1, 2019(JavaScript required) .
- ↑ a b c Material data for acetaldehyde at Celanese Chemicals. As of December 1999.
- ↑ a b c d e Entry on acetaldehyde in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dipole Moments, pp. 9-52.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-4.
- ↑ Entry on acetaldehyde in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on November 15, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values , accessed on July 19, 2019.
- ↑ Van M. Sim, MD; Richard E. Pattle, MA: Effect of possible smog irritants on human subjects , JAMA, Journal of the American Medical Association. 1957; 165 (15): 1908-1913. doi : 10.1001 / jama.1957.02980330010003 , PMID 13480837 .
- ^ National Technical Information Service. Vol. OTS0534485.
- ↑ Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 25 (11), p. 57, 1981.
- ^ Agents and Actions, A Swiss Journal of Pharmacology. Vol. 4, p. 125, 1974.
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-22.
- ↑ a b c d e Ullmann's Encyclopedia of Industrial Chemistry, 2007.
- ↑ test.de: Natural mineral waters: Bad grades for discounters , July 24, 2008.
- ↑ T. Karl, AJ Curtis, TN Rosenstiel, RK Monson, R. Fall: Transient releases of acetaldehyde from tree leaves - products of a pyruvate overflow mechanism ?. In: Plant Cell And Environment , Vol. 25, Issue 9, 2002, pp. 1121-1131, doi : 10.1046 / j.1365-3040.2002.00889.x .
- ^ Henri A Favre, Warren H Powell: Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 . Ed .: The Royal Society of Chemistry. Cambridge 2014, ISBN 978-0-85404-182-4 , pp. 908 , doi : 10.1039 / 9781849733069-FP001 .
- ↑ Experimental School Chemistry of Secondary School II, Aulis-Deubner Verlag GmbH & Co. KG, Vol. 1–12, Aldehyde, p. 91.
- ↑ Entry on metaldehyde. In: Römpp Online . Georg Thieme Verlag, accessed on October 31, 2014.
- ↑ FA Cotton, JN Francis, BA Frenz, M. Tsutsui: Structure of a dihapto (vinyl alcohol) complex of platinum (II) , in: Journal of the American Chemical Society , 1973 , 95 , pp. 2483-2486. doi : 10.1021 / ja00789a011 .
- ↑ Moritz Brandt, Venkata Garlapati u. a .: NOX2 amplifies acetaldehyde-mediated cardiomyocyte mitochondrial dysfunction in alcoholic cardiomyopathy. In: Scientific Reports. 6, 2016, p. 32554, doi : 10.1038 / srep32554 .
- ↑ Hans-Dieter Jakubke, Ruth Karcher (Ed.): Lexicon of Chemistry , Spectrum Academic Publishing House, Heidelberg, 2001.