Pentaerythritol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
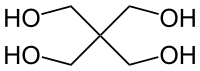
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Pentaerythritol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 12 O 4 | ||||||||||||||||||
| Brief description |
white, crystalline powder with a sweet taste |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 136.15 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.396 g cm −3 |
||||||||||||||||||
| Melting point |
258 ° C |
||||||||||||||||||
| boiling point |
276 ° C (30 h Pa ) |
||||||||||||||||||
| Vapor pressure |
2.53 · 10 −8 Torr (25 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Pentaerythritol (according to IUPAC nomenclature 2,2-bis (hydroxymethyl) -1,3-propane-diol) is a tetravalent alcohol . The prefix penta (five) indicates the 5 carbon atoms; Erythritol on the likewise tetravalent alcohol.
Extraction and presentation
The technical production takes place through the conversion of formaldehyde with acetaldehyde through a triple aldol reaction with a subsequent crossed Cannizzaro reaction . A molar excess of formaldehyde is used in order to avoid the formation of dipentaerythritol . Pentaerythritol was discovered by Bernhard Tollens in 1882 when he mixed an aqueous solution of formaldehyde and acetaldehyde with barium hydroxide .
properties
Physical Properties
Pentaerythritol is a white, crystalline powder with a sweet taste . It is well soluble in boiling water and moderately soluble in cold water. It is sparingly soluble in ethanol and insoluble in benzene , carbon tetrachloride , ether and petroleum ether .
Chemical properties
The four hydroxyl groups essentially enter into the reactions typical of alcohols, such as esterification with acids . The esterification of pentaerythritol with nitric acid , not quite correctly called nitration, results in pentaerythritol tetranitrate ( nitropenta ).
use
PE is mainly used for the production of alkyd resins as well as plasticizers and emulsifiers, and also for the production of the explosives pentaerythritol trinitrate and nitropenta (pentaerythritol tetranitrate).
literature
- Paul Karrer: Textbook of Organic Chemistry . 10th edition, Georg Thieme Verlag, Stuttgart 1948, p. 335
Individual evidence
- ↑ a b c d e Entry on pentaerythritol. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ a b c d e Entry for CAS no. 115-77-5 in the GESTIS substance database of the IFA , accessed on January 15, 2016(JavaScript required) .
- ^ Entry on pentaerythritol in the Hazardous Substances Data Bank , accessed January 15, 2016.
