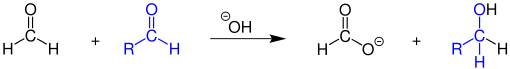
Cannizzaro reaction
| Aldehydes |
 2,2-Dimethylpropaldehyde and benzaldehyde are suitable starting materials for the Cannizzaro reaction, since they do not contain a hydrogen atom on the α-carbon atom next to the carbonyl group. |
 Aldehydes with blue hydrogen atoms on the α-carbon atom next to the carbonyl group. These aldehydes can be enolized and react to aldols under alkaline conditions ; a Cannizzaro reaction does not take place. |
The Cannizzaro reaction is a name reaction from organic chemistry . It was named after its discoverer - the Italian chemist Stanislao Cannizzaro (1826–1910). Aldehydes which do not have a hydrogen atom bonded to the carbon atom in the α-position to the carbonyl group react in this reaction in a strongly basic medium to form the salt of a carboxylic acid and an alcohol . A strongly basic medium is, for example, concentrated sodium hydroxide solution , which creates the sodium salt a carboxylic acid and the alcohol.
If an α-hydrogen atom is present in the aldehyde, the aldol reaction takes place preferentially .
Overview reaction
In the reaction, aldehydes which do not have a hydrogen atom in the α-position to the aldehyde carbon atom disproportionate in the presence of strong bases .
As oxidation product of the corresponding salt is formed of a carboxylic acid , as a reduction product of alcohol . The residues on the aldehyde can be alkyl or aryl groups. The alkyl group must not have an α-hydrogen atom. In addition, the residues can be identical.
Reaction mechanism
In this mechanism, the Cannizzaro reaction is explained using a general aldehyde without an α-hydrogen atom. The reaction takes place, for example, in an aqueous solution of concentrated sodium hydroxide solution.
A hydroxide ion attacks the aldehyde 1 nucleophilically, so that the simply deprotonated diol 2 is formed. This is deprotonated to dianion 3 by a further hydroxide ion . With the formation of a carbonyl group , a hydride ion is split off, which in turn attacks an aldehyde on the carbonyl group. The intermolecular hydride is determined by the electron-donating effect of O - - substituents possible. The hydride transfer must take place within a molecular complex, since the hydride ions would otherwise react with the protons always present in water to form hydrogen . The alcoholate 4 and carboxylate 5, corresponding to the aldehyde, are formed as intermediate products . The intermediate product 4 is protonated by the solvent in an acid-base reaction to form alcohol 6 . The carboxylate 5 can now precipitate with the sodium ion of the sodium hydroxide solution as the sodium salt of the corresponding carboxylic acid (not always).
Crossed Cannizzaro reaction
Since carboxylic acids are easier to synthesize in other ways, the Cannizzaro reaction is usually used to obtain the alcohol. Since half the alcohol and the other half the carboxylic acid are always formed from one mole of an aldehyde, the atom economy of the classic Cannizzaro reaction is low (only a maximum of 50% of the aldehyde is reduced to the desired alcohol, the other 50% is oxidized). This is why the “crossed Cannizzaro reaction” is used more frequently, in which formaldehyde is used as an additional component .
The formaldehyde is attacked by the base and then releases a hydride ion to the second aldehyde, which is reduced to alcohol, while the formaldehyde is oxidatively converted into a formate (salt of formic acid ):
This measure increases the yield of alcohol. For example, the tetravalent alcohol pentaerythritol , which has four hydroxyl groups, is produced by a triple aldol reaction from formaldehyde and acetaldehyde with a subsequent cross Cannizzaro reaction.
literature
- Organikum. 16th edition, VEB Deutscher Verlag der Wissenschaften, Berlin 1985, ISBN 3-326-00076-6 , p. 490.
- TA Geissman: The Cannizzaro Reaction. In: Organic Reactions. 2, No. 3, 1944, pp. 94-113, doi: 10.1002 / 0471264180.or002.03
Web links
Individual evidence
- ↑ S. Cannizzaro: About the alcohol corresponding to benzoic acid . In: Justus Liebig's Annals of Chemistry . tape 88 , no. 1 , 1953, p. 129-130 , doi : 10.1002 / jlac.18530880114 .
- ↑ K. List, H. Limpricht: About the so-called Benzoëoxide and some other paired compounds . In: Justus Liebig's Annals of Chemistry . tape 90 , no. 2 , 1854, p. 190-210 , doi : 10.1002 / jlac.18540900212 .
- ↑ a b L. Kürti , B. Czakó: Strategic Applications of Named Reactions in Organic Synthesis . Elsevier Academic Press, Burlington / San Diego / London 2005, ISBN 978-0-12-429785-2 , p. 74.
- ↑ Peter Sykes: Reaction Mechanisms - An Introduction . 8th edition. VCH, Weinheim 1982, ISBN 3-527-21090-3 S, pp. 244-246.
- ↑ Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken NJ 2009, ISBN 978-0-471-70450-8 , p. 1477.