Disproportionation
In a chemical reaction known as disproportionation (also called dismutation ), an intramolecular redox reaction reacts with a component of a compound that is present several times - e.g. B. One type of atom - both as an oxidizing agent and as a reducing agent . After the disproportionation reaction has elapsed, this constituent (atomic type), which was previously in a medium oxidation state, is present in both an increased oxidation state and an oxidation state that is reduced to the same extent. The type of atom in question is thus partly oxidized and reduced to the same extent . The reverse reaction is called comproportionation or synproportionation .
Examples
- Simple examples of disproportionation: reaction of elemental chlorine in sodium hydroxide solution . In the course of the reaction, one chlorine atom of the chlorine molecule (oxidation number 0) is reduced to the chloride anion (oxidation number −I), the other chlorine atom is oxidized to (hypochlorite anion oxidation number + I).
- Chlorine reacts in cold sodium hydroxide solution ( exothermic ) to form sodium chloride , sodium hypochlorite and water .
- In warm sodium hydroxide solution, the sodium hypochlorite formed can be further oxidized to sodium chlorate with excess chlorine .
- This reaction is also used in water treatment in swimming pools. When chlorine is introduced into water, there is an equilibrium of chlorine, hypochlorous acid and hydrochloric acid , depending on the pH value .
- Depending on the pH value , the hypochlorous acid partially dissociates into oxonium and hypochlorite ions:
- Reaction when nitrogen dioxide is dissolved in water:
- In two molecules of nitrogen dioxide , the N atoms (oxidation number IV) are oxidized to (N oxidation number + V). in the third molecule the N atom is reduced to nitrogen monoxide (N oxidation number + II).
- A more complicated example of a disproportionation reaction: disproportionation of potassium chlorate (chlorine oxidation state V) with the formation of potassium chloride (chlorine oxidation state -I) and potassium perchlorate (chlorine oxidation state VII). This reaction must proceed according to the following reaction equation, because only then is the balance with regard to the increase or decrease in the oxidation number of the chlorine atoms balanced: 3 chlorine atoms experience an increase in their oxidation number by 2 levels and accordingly 1 chlorine atom experiences a decrease in it Oxidation number around 6 levels.
- Potassium chlorate reacts to form potassium chloride and potassium perchlorate (exothermic).
Radical disproportionation
- A special case is radical disproportionation in organic chemistry : In this reaction, the oxidation state of the central carbon atom in the radical on the left increases to the same extent as the oxidation state of the central carbon atom in the radical on the right decreases. From a formal point of view, an H atom is transferred from the left to the right radical.
Cannizzaro reaction
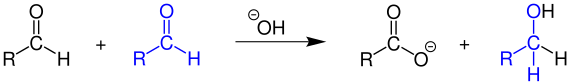
In the Cannizzaro reaction, aldehydes that do not have a hydrogen atom in the α position to the aldehyde carbon atom disproportionate in the presence of strong bases such as concentrated sodium hydroxide solution . As oxidation product of the corresponding salt is formed of a carboxylic acid (eg. Sodium salt , as a) reduction product of the alcohol :
However, if an α-hydrogen atom is present in the aldehyde, the aldol reaction is preferred.
Web links
Individual evidence
- ↑ a b entry on disproportionation . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.D01799 Version: 2.1.5.
- ^ Fair, GM, J. Corris, SL Chang, I. Weil, and RP Burden. 1948. The behavior of chlorine as a water disinfectant. J. Am. Water Works Assoc. 40: 1051-1061.
- ^ Organikum , Wiley-VCH Verlag GmbH, 23rd edition, 2009, p. 194, ISBN 978-3-527-32292-3 .
- ^ László Kürti , Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis ; Elsevier Academic Press, Burlington-San Diego-London 2005, 1st edition; ISBN 0-12-369483-3 , p. 74.