Potassium chlorate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Potassium chlorate | |||||||||||||||
| other names |
|
|||||||||||||||
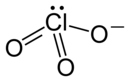
| Molecular formula | KClO 3 | |||||||||||||||
| Brief description |
colorless, glossy, monoclinic panels |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 122.55 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.32 g cm −3 |
|||||||||||||||
| Melting point |
368 ° C |
|||||||||||||||
| boiling point |
Decomposes at 370 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Potassium chlorate ( molecular formula KClO 3 ) is the potassium salt of chloric acid (HClO 3 ).
Potassium chlorate is a white stable salt that makes a colorless solution in water. The compound has a strong oxidizing effect and is used, among other things, to make matches , primers and other pyrotechnic products.
Manufacturing
Potassium chlorate is obtained by electrolysis of a sodium chloride solution and subsequent precipitation of the resulting sodium chlorate with potassium chloride . In electrolysis, the cathode ( steel ) and anode (activated titanium ) are not separated by a membrane, as in chlor-alkali electrolysis , but are placed close to one another. As a result, the chlorine formed as an intermediate in the electrolysis comes into contact with the sodium hydroxide solution and forms sodium hypochlorite :
At higher temperatures this disproportionates to chloride and chlorate:
During the electrolysis of a potassium chloride solution, the potassium chlorate is formed directly, with the following reactions taking place:
At the cathode:
At the anode:
In the past, potassium chlorate was produced by introducing chlorine into a potassium hydroxide solution. Potassium chloride and potassium hypochlorite were initially formed in equal parts. In the heat generated during the reaction, the hypochlorite disproportionates to two parts chloride and one part chlorate. Basically, the same reactions occur as in the variant mentioned above, but more expensive starting materials (chlorine gas and potassium hydroxide) are required, but no electricity. The overall reaction equation is:
This shows that only one sixth of the chlorine used is present in the chlorate yield.
properties
Potassium chlorate is poor in cold water, more soluble in warm water and, unlike sodium chlorate, is not hygroscopic. When it crystallizes out of an aqueous solution, it forms brilliantly shiny and shimmering flat crystals. However, if it is precipitated, for example by adding a potassium salt to a sodium chlorate solution, it results as an extremely fine, far less shiny powder. It is an oxidizing agent , when heated above the melting point it disproportionates to potassium perchlorate and potassium chloride:
When heated above 550 ° C it breaks down completely into oxygen and potassium chloride. This decomposition takes place at 150 to 200 ° C when manganese dioxide (brownstone) is added as a catalyst .
Mixtures with oxidizable substances such as sulfur , phosphorus , iodine and carbon , which can explode through friction, impact or impact , are very explosive . The most explosive mixtures are with red phosphorus and are considered dynamite-like ( Armstrong mixture ) when mixed in the finest way . In technology, quantities in the single-digit milligram range are therefore only mixed wet and used with binding agent for popping corks and ignition platelets .
- Potassium chlorate reacts with sulfur to form potassium chloride and sulfur dioxide .
- The reaction of potassium chlorate and carbon results in potassium chloride and carbon dioxide .
The maximum therapeutic, i.e. H. non-toxic, dose is 1 g for an adult. Above that it is a strong blood and kidney toxin. The lethal dose is 5–15 g. A possible antiseptic effect is now usually assessed with skepticism and even doubted and it may only be used to a limited extent due to its toxicity.
use
Potassium chlorate is used in the laboratory to produce oxygen. It is used for the production of light and toy ammunition, but above all in the fuses of matches .
In fireworks today it is replaced by potassium perchlorate where possible , which is safer to handle. Nevertheless, potassium chlorate is still required for certain particularly color-intensive effects. It was also used to produce explosives . Chloratite contains z. B. about 90% potassium chlorate, 10% hydrocarbons and an addition of wood flour. These friction-sensitive mixtures have largely been replaced by oxidizing agents that are safe to handle, in particular by potassium perchlorate, which is significantly more stable and at least as effective when mixed with fuels. If a weaker oxidizing agent is needed, e.g. B. for the black powder in propellants of a fireworks rocket, or pyrotechnic ball and cylinder bombs, the well-known potassium nitrate is mostly used today.
It was not without risk that they were used for “ snap peas ”, where a mixture of potassium chlorate and red phosphorus was formed into balls together with gum arabic and then dried. The mixture of potassium chlorate and red phosphorus is also known as Armstrong's mixture and repeatedly leads to serious injuries when handling it if the great sensitivity of this mixture to shock, friction or electrostatics is underestimated.
At the same time, this highly dangerous and unpredictable Armstrong mixture is used to ignite the very safe safety matches. Here it is freshly formed in traces by the rubbing of the ignition head containing chlorate on the friction surface with red phosphorus and explodes immediately. In these quantities this leads to a safe inflammation of the head. The distribution of the ignition mixture on the match head and friction surface is the decisive safety advantage of this construction, which was invented around 1844 by the Swedish chemist Gustaf Erik Pasch , because such a match can hardly ignite without a friction surface. The high ignition energy now available made the very toxic white phosphorus , which had been used in match heads, unnecessary.
In general, potassium chlorate, as a pure substance, should also be treated with caution, since even minor impurities, especially with red phosphorus, but also sulfur or metal powders, can significantly increase the potential risk of involuntary, spontaneous self-ignition.
Potassium chlorate is part of the " Dutch bath ", an etching liquid for copper plates made of 88% water, 10% conc. Hydrochloric acid and 2% potassium chlorate, which is used to make etchings .
In the past, potassium chlorate was thought to have an antiseptic effect and was therefore used in gargles and mouthwashes. Today, this effect is mostly viewed with skepticism and even questioned. There are only a few gargles left that contain potassium chlorate, as its toxicity means that its use is restricted.
With 'Kalium chloratum', which u. A. is used as an active ingredient in homeopathic finished medicinal products such as ointments and globules, contrary to the Latin name, it is not potassium chlorate, but potassium chloride (= KCl - also known as 'salt substitute').
In the past, sodium and potassium chlorate were used and sold as weed killers under the trade name UnkrautEx . Due to the potential for danger and abuse, it has not been available in Germany for several years.
Individual evidence
- ↑ a b Entry on potassium chlorate. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ a b c d e f g Entry on potassium chlorate in the GESTIS substance database of the IFA , accessed on December 7, 2019(JavaScript required) .
- ↑ Entry on Potassium chlorate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- ↑ a b Alexander P. Hardt: Pyrotechnics , Pyrotechnica Publications, Post Falls Idaho USA 2001, ISBN 0-929388-06-2 , pp. 74 ff.
- ↑ Hermann Ammon (Ed.): Hunnius Pharmaceutical Dictionary . 11th edition, de Gruyter, Berlin 2014, ISBN 978-3-11-030990-4 . Entry: potassium chlorate
- ↑ Takeo Shimizu: Fireworks - The Art, Science and Technique, Pyrotechnica Publications, Midland Texas USA, 4th Edition, p. 53 ff., P. 92 ff. ISBN 0-929388-05-4
literature
- John BC Kershaw: The Electrolytic Chlorate Industry . Verlag von Wilh. Knapp, Hall 1905 (Reprint 2010, Survival Press)














