Potassium nitrate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
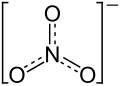
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Potassium nitrate | |||||||||||||||
| other names | ||||||||||||||||
| Molecular formula | KNO 3 | |||||||||||||||
| Brief description |
colorless to white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 101.11 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.11 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
334 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| solubility |
good in water (316 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Potassium nitrate or bengal nitrate , often referred to as saltpeter in common parlance , in particular as potassium nitrate (formerly also “salniter”: 'purified saltpeter'), is the potassium salt of nitric acid .
properties
Potassium nitrate forms colorless crystals, which dissolve in water under strong cooling. It is therefore much more soluble in warm water than in cold water. Up to 130 g of potassium nitrate can be dissolved in one liter of water at 0 ° C, and up to 2455 g of potassium nitrate at 100 ° C.
Potassium nitrate decomposes to potassium nitrite and oxygen when heated :
It is an excellent oxidizing agent at elevated temperatures . Charred residue in glassware dissolves quickly in molten potassium nitrate.
Potassium nitrate is significantly less hygroscopic than many other nitrates, e.g. B. Sodium Nitrate .
Extraction
Natural occurrence
Potassium nitrate (min. "Nitrocalite"; Latin earlier sal nitri : also impure saltpeter ) occurs as efflorescence on soils. The deposits in China and Southeast Asia were of economic importance, where in the first half of the 19th century more than 10,000 tons of saltpeter were extracted annually by leaching such soils.
Bacterial nitrification of nitrogen-rich organic waste
From the end of the 14th to the 19th century, saltpeter was produced in Europe with the help of bacteria and atmospheric oxygen in order to be independent of the import of this raw material, which is essential for war purposes (see “History”). Nitrogen-rich organic waste (manure and urine) is mixed with lime and wood ash ( potash ) and left to rot in loose, air-permeable piles of earth. The nitrogen compounds are converted into nitrates by bacteria. After two years the mass is leached with water. Potash (potassium carbonate) is added to the raw liquor (in particular consisting of sodium nitrate or sodium nitrate), which converts calcium and magnesium nitrate into potassium nitrate and poorly soluble alkaline earth carbonate . The potassium nitrate is obtained by evaporating the filtered alkali and is purified by recrystallization.
Conversion nitrate
From the middle of the 19th century to around 1920, the conversion of Chile's nitrate with potassium chloride from German potash mining was the most important process for the production of potassium nitrate:
The slight increase in the solubility of sodium chloride with temperature is used: the mother liquor from the KNO 3 crystallization in the previous cycle is heated and pure sodium nitrate and potassium chloride (in stoichiometric amounts) are added. The mixture is concentrated with the addition of a little soda at 100 ° C., sodium chloride and impurities ( alkaline earth carbonates ) precipitating and being filtered off. The filtrate is diluted again with the evaporation condensate in order to avoid the precipitation of sodium salts during cooling, filtered until clear, then cooled to 5 ° C. for the crystallization of the potassium nitrate and centrifuged. The deposited potassium nitrate is recrystallized for technical purposes.
Synthetic from nitric acid
Today potassium nitrate is produced synthetically from nitric acid (see illustration ).
presentation
There are many - theoretical and practicable - ways of representing potassium nitrate:
- almost all through salt formation reactions :
The formed ammonium carbonate decomposes starting at about 60 ° C to ammonia , carbon dioxide and water .
use
- Potassium nitrate is used to preserve food ( curing salt E 252).
- It is the main ingredient in black powder .
- A mixture of 24% boron + 71% KNO 3 + 5% binder serves as a reliable ignition mixture that burns even at very low temperatures (−196 ° C).
- A mixture of 60% NaNO 3 + 40% KNO 3 melts at 222 ° C and is used as a heat transfer medium in solar thermal power plants. This molten salt is chemically stable up to 590 ° C, has a high specific heat capacity of 1.55 kJ / (kg K), a density of 1.79 g / cm 3 and is as thin as water ( viscosity : 2.1 mPa s). It wets metal surfaces very easily, which can lead to sealing problems if the construction and material selection are unsuitable. Stainless steel is largely resistant to molten saltpeter (removal rate: 6–15 µm / year at 570 ° C). The heat transfer coefficient on the pipe with a turbulent flow is about 6000 W / K · m 2 . Due to its high heat capacity (2.8 MJ / (K · m 3 )), molten nitrate is also suitable as a heat storage medium. The melting temperature can be further reduced by adding sodium nitrite . A salt mixture, known as HiTec , consisting of 53% KNO 3 + 40% NaNO 2 + 7% NaNO 3 melts at 140 ° C and has particularly favorable properties as a heat transfer medium when the toxicity of sodium nitrite is irrelevant.
- Saltpetre baths are used for the heat treatment of wrought aluminum alloys with a magnesium content of up to 10%. The maximum permissible temperature of the molten salt depends on the magnesium content; it drops from 550 ° C with 0.5% Mg to 380 ° C with 10% Mg.
- in fertilizer
- in toothpaste for sensitive teeth
- Qualitative detection of manganese and chromium in the soda-saltpeter melt.
history
As early as the 11th century, in the book of Marcus Graecus , which also mentions the black powder mixture for the first time , saltpeter is mentioned as a new substance that is scraped off the earth and stones. The book on mounted combat and the use of war machines by Hasan al-Rammah ( Al-Furusiyya wa al-Manasib al-Harbiyya ), dating from the end of the 13th century, already contains several regulations for cleaning saltpetre with wood ash and for making incendiary devices and fuel for missiles.
Saltpetre was initially imported from India ; Venice made high profits from the middlemen. With increasing demand and for reasons of independence, from the end of the 14th century governments promoted their own mining of saltpeter and secured all rights of manufacture, import and use by means of draconian laws through a "saltpeter shelf". Due to the rapid release of oxygen, saltpetre was the basis for the sudden combustion of sulfur and charcoal in gunpowder and therefore, as a chronically scarce substance, the strategic raw material for six centuries.
In Thuringia there were nine saltpeter works in the 16th century . The banks of the Vltava near Prague were covered with "sanitary benches", the city of Halle granted a concession to extract saltpetre from the garbage dumps. The increasing demand for saltpetre was partly covered by further imports, especially from India, and by our own plants.
From the end of the 14th century, a systematic cultivation of saltpetre gardens took place. Animal waste (manure, excrement , urine and blood ) was filled with calcareous earth, earth from cemeteries or slaughterhouses or from moors and with lime , rubble and ashes in pits or piled in piles and occasionally doused with manure or urine. As a result of the decomposition, so much saltpeter was formed after one or two years that it could be washed out of the earth. The yield was about 6: 1, 1 kg of saltpeter was obtained from 6 kg of nitric earth.
Saltpeter as a special and very unpopular profession was allowed to enter properties at any time and look for saltpeter there. This was true even for churches in the 17th and 18th centuries, with the exception of the times of church services. In Sweden the farmers even had to pay some of their taxes in saltpeter.
From 1879 to 1884 Chile waged the saltpeter war against its neighbors Peru and Bolivia in order to gain sole ownership of the huge desert deposits of sodium nitrate ("caliche"), which could immediately be converted into potassium nitrate with potassium salts. This conversion process was replaced from 1916 by the Haber-Bosch process , the production of ammonia from air and water with subsequent conversion to nitric acid.
Individual evidence
- ↑ Entry on E 252: Potassium nitrate in the European database on food additives, accessed on June 27, 2020.
- ↑ Entry on POTASSIUM NITRATE in the CosIng database of the EU Commission, accessed on February 25, 2020.
- ↑ a b c d e f g Entry on potassium nitrate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on potassium nitrate. In: Römpp Online . Georg Thieme Verlag, accessed on May 30, 2014.
- ^ Wilhelm Hassenstein: The fireworks book from 1420. 600 years of German powder weapons and gunsmithing. Reprint under the title Büchsenmeysterei with translation into standard German and explanations, Munich 1941, pp. 40, 53, 59, 67, 106 and 114.
- ↑ Irene Strube, Rüdiger Stolz, Horst Remane: History of Chemistry: An Overview from the Beginnings to the Present. Berlin 1986, p. 46.
- ↑ M. Baetz: Black Powder for Survival Volume 1 Improvisation of Black Powder and Similar Mixtures, p. 70, 2005, Fuldaer Verlagsanstalt.
- ↑ Potassium nitrate . Drugs.com. Retrieved November 15, 2015.
literature
- M. Baetz: Black powder for survival Volume 1 Improvisation of black powder and similar mixtures, p. 70, 2005, Fuldaer Verlagsanstalt
- RH Perry: Chemical Engineers' Handbook . 4th ed., McGraw-Hill Book Company, New York, 1963, pp. 9-77
- GH Janz et al .: Physical Properties Data Compilations Relevant to Energy Storage II. Molten Salts , NSRDS, April, 1979
- J. Gartz: Cultural history of explosives . ES Mittler & Sohn, Hamburg, 2006
- Seel, Wolfgang: Prussian-German powder history. Deutsches Waffen-Journal 19 (1983) No. 3, pp. 294-301, No. 4, pp. 462-465, No. 5, pp. 588-592, No. 7, pp. 862-867, No. 8, pp. 1020–1023, No. 9, pp. 1144–1146, 44 figs.
- Seel, Wolfgang: Old Prussian saltpeter industry. Waffen- und Costumekunde B. 25 (1983) H. 1, pp. 31–41, 4 figs.













