Ammonium carbonate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
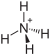 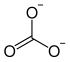
|
||||||||||
| General | ||||||||||
| Surname | Ammonium carbonate | |||||||||
| other names |
|
|||||||||
| Molecular formula | CH 8 N 2 O 3 | |||||||||
| Brief description |
hygroscopic colorless solid with an ammonia-like odor |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | ||||||||||
| Physical state |
firmly |
|||||||||
| density |
1.13 g cm −3 (at 25 ° C ) |
|||||||||
| Melting point |
Decomposition from 58 ° C |
|||||||||
| Vapor pressure |
69 h Pa (20 ° C) |
|||||||||
| solubility |
good in water (320 g l −1 at 20 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| Thermodynamic properties | ||||||||||
| ΔH f 0 |
−942 kJ mol −1 |
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Ammonium carbonate , (NH 4 ) 2 CO 3 · H 2 O, forms water-soluble, colorless, cubic crystals. From 58 ° C it completely breaks down into ammonia , carbon dioxide and water .
properties
The salt forms a colorless powder that smells slightly of ammonia. It has a density of 1.6 g · cm −3 (at 20 ° C). In aqueous solution it has a weakly basic reaction , an equilibrium is established between ammonia NH 3 , ammonium ions NH 4 + , carbonate ions CO 3 2− , hydrogen carbonate ions HCO 3 - and carbon dioxide CO 2 . The pH value of a 10% aqueous solution at 25 ° C is 9.4.
The salt reacts with water to form hydrogen carbonate and hydroxide ions . An aqueous solution of ammonium carbonate is therefore only stable in a neutral and weakly basic environment - carbon dioxide gas escapes in acidic solutions, and ammonia gas in concentrated alkalis.
Ammonium carbonate decomposes in air; the reaction takes place violently when it is hot.
Extraction and presentation
Ammonium carbonate can be prepared by reacting carbon dioxide with ammonia in an aqueous solution.
A further possibility of presentation results from heating calcium carbonate together with ammonium sulphate .
In the latter method, in addition to the desired ammonium carbonate, ammonium hydrogen carbonate and ammonium carbamate also sublime , while calcium sulfate remains as a solid .
use
Ammonium carbonate is used in the synthesis of heterocycles and as an additive in photographic developers. It is also used in dyeing as a stain, as a spotting agent and as a carbon dioxide developer in extinguishing devices.
It is also used as a raising agent (as a component of deer horn salt ). In the EU it is approved as a food additive with the number E 503i .
It was also previously held under the nose as a smelling salt to stimulate dizziness and fainting spells.
Ammonium carbonate is often used in inorganic chemistry for qualitative analysis in order to precipitate the alkaline earth metal cations of barium , strontium and calcium as a group in the cation separation process from an unknown sample , to separate them and to identify them with the help of detection reactions.
It is also used for the production of catalysts, foams, hair treatment products and casein colors and glues.
Individual evidence
- ↑ Entry on E 503: Ammonium carbonates in the European database for food additives, accessed on June 27, 2020.
- ↑ a b c d Data sheet ammonium carbonate (PDF) from Merck , accessed on January 19, 2011.
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons . William Andrew, 2014, ISBN 978-0-323-29060-9 , pp. 265 ( limited preview in Google Book search).
- ↑ Entry on ammonium carbonate. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b c d Entry on ammonium carbonate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ PAETEC Formula Collection Edition 2003, p. 116.
- ↑ Brochure Kremer Pigments ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.

![{\ mathrm {\ \! \ {\ Biggr]} _ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/574adab1409cb81da6c38bb738ad111e61bbb2d9)
![{\ mathrm {\ \! \ {\ Biggr]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c9fd9553a0b623f5f669641be907d9186e35b739)


