Alkaline earth metals
|
Position in the periodic table
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| group | 2 |
| period | |
| 2 |
4 Be |
| 3 |
12 mg |
| 4th |
20 approx |
| 5 |
38 Sr |
| 6th |
56 Ba |
| 7th |
88 Ra |
When alkaline earth metals are chemical elements beryllium , magnesium , calcium , strontium , barium and radium from the 2nd main group of the Periodic Table referred to. They are shiny, reactive metals that have two electrons in their valence shell . Radium is a radioactive intermediate product of natural decay series . The name is derived from the two neighboring main groups, the alkali metals , with which they have the formation of strong bases in common, and the earth metals , with which they have in common that they are poorly water-soluble .
properties
The typical alkaline earth metals are calcium , strontium and barium . Beryllium resembles the other alkaline earth metals very little, so that beryllium is also assigned to the zinc group . The alkaline earth metals are light metals that have a metallic sheen. The gloss disappears quickly in the air because the metal is oxidized . Beryllium and magnesium are quite stable in dry air . Magnesium reacts similarly to lithium with the nitrogen in the air. That is why one speaks of the oblique relationship to the element lithium. Alkaline earth metals conduct the electrical current , and each have two outer electrons . In compounds they occur almost exclusively as divalent cations .
The typical alkaline earth metals and their salts have a specific flame color :
- Calcium and its salts color the flame orange-red (622 and 553 nm).
- Strontium and its salts color the flame red (675 and 606 nm).
- Barium and its salts color the flame green (524 and 514 nm).
Because of this flame color, alkaline earth metal compounds are used for fireworks .
Physical Properties
With increasing atomic number , atomic mass , atomic radius and ionic radius grow .
Calcium has the lowest density with 1550 kg / m³. It rises upwards and especially downwards, with radium reaching the maximum value of 5500 kg / m³.
The Mohs hardness of beryllium is 5.5 in the middle range. The other elements of the 2nd main group have low hardnesses, which decrease with increasing atomic number.
The first three alkaline earth metals, especially beryllium and calcium, are very good electrical conductors . Although the other elements of this main group are by no means bad leaders, the difference is considerable.
The 1st ionization energy falls with increasing atomic number from 9.322 eV for beryllium to 5.212 eV for barium. At 5.279 eV, radium again has a slightly increased value.
The electronegativity drops from 1.57 for beryllium to 0.9 for radium.
| element | beryllium | magnesium | Calcium | strontium | barium | radium |
|---|---|---|---|---|---|---|
| Melting point (1013 hPa) | 1560 K
(1287 ° C) |
923 K
(650 ° C) |
1115 K
(842 ° C) |
1050 K
(777 ° C) |
1000 K
(727 ° C) |
973 K
(700 ° C) |
| Boiling point (1013 hPa) | 3243 K
(2969 ° C) |
1383 K
(1110 ° C) |
1760 K
(1487 ° C) |
1653 K
(1380 ° C) |
1910 K
(1637 ° C) |
2010 K
(1737 ° C) |
| Density (20 ° C, 1013 hPa) | 1.848 g / cm 3 | 1.738 g / cm³ | 1.55 g / cm 3 | 2.63 g / cm 3 | 3.62 g / cm 3 | 5.5 g / cm³ |
| Mohs hardness | 5.5 | 2.5 | 1.75 | 1.5 | 1.25 | |
| Electric conductivity | 25 x 10 6 S / m | 22.7 x 10 6 S / m | 29.4 x 10 6 S / m | 7.41 x 10 6 S / m | 2.94 x 10 6 S / m | 1 x 10 6 S / m |
| Atomic mass | 9,012 u | 24,305 u | 40,078 u | 87.62 u | 137,327 u | 226,025 u |
| Electronegativity | 1.57 | 1.31 | 1.00 | 0.95 | 0.89 | 0.9 |
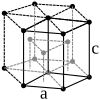
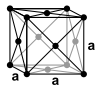
| structure |
|

|
|

|

|

|
| Crystal system | hexagonal | hexagonal | Cubic area-centered | Cubic area-centered | body-centered cubic | body-centered cubic |
Electron configuration
The electron configuration is [ X ] y s ². The X stands for the electron configuration of the noble gas , which is one period higher , and the period in which the element is located must be used for the y .
The electron configurations for the individual elements are:
- Beryllium: [ He ] 2s²
- Magnesium: [ Ne ] 3s²
- Calcium: [ Ar ] 4s²
- Strontium: [ Kr ] 5s²
- Barium: [ Xe ] 6s²
- Radium: [ Rn ] 7s²
The oxidation state is +2, as the two electrons in the outer shell can easily be given up. Me 2+ ions have a noble gas configuration .
Reactions
The alkaline earth metals reach noble gas configurations by releasing their two outer electrons . Compared to the alkali metals , however, they are less reactive because it requires a higher ionization energy to split off two outer electrons than one, as is the case with the alkali metals. This can be justified with the fact that the alkaline earth metals have a higher nuclear charge and thus correspondingly smaller atomic radii than the alkali metals.
Within the group of alkaline earth metals, the reactivity increases from top to bottom, because between the outer electrons and the atomic nucleus there are more and more full electron shells and so the distance between the outer electrons and the core increases. This means that these are less strongly attracted to the atomic nucleus and can therefore be split off more easily.
The alkaline earth metals easily give off their two outer electrons , producing doubly positively charged ions , and are therefore base metals that are oxidized in the air . Beryllium and magnesium , however, form stable oxide layers and are thereby passivated, ie only their surface is oxidized. This passivation also means that water attacks beryllium and magnesium only slowly. Calcium , strontium and barium, on the other hand, react with water to form hydroxides , producing hydrogen . Like the alkali metals , the alkaline earth metals are also base formers . Otherwise the alkaline earth metals react well with non-metals , e.g. B. with oxygen or with the halogens .
In the following reaction equations , Me stands for an alkaline earth metal.
- Barium also forms barium peroxide .
The reactivity , which increases with the atomic number, can be clearly observed in the reaction behavior:
- Resistance to air :
- Beryllium is stable in dry air at room temperature because it is covered by a passivating oxide layer.
- Magnesium is also passivated in air, but thin ribbons and foils can easily be ignited.
- Calcium , strontium , barium and radium accumulate quickly in dry air and, in finely divided form, are self-igniting .
- Reaction with hydrogen at high temperatures :
-
Reaction with water :
- Like aluminum, beryllium is passivated in water.
- Magnesium is also passivated , but the passivation layer dissolves in hot water.
- The other alkaline earth metals react violently with water at room temperature .
links
Beryllium is the only alkaline earth metal that forms predominantly covalent bonds. The other elements of the 2nd main group occur almost exclusively as Me 2+ ions. The table provides a rough overview of the most important connections:
| beryllium | magnesium | Calcium | strontium | barium | |
|---|---|---|---|---|---|
| Oxides | BeO | MgO | CaO | SrO | BaO |
| Hydroxides | Be (OH) 2 | Mg (OH) 2 | Ca (OH) 2 | Sr (OH) 2 | Ba (OH) 2 |
| Fluoride | BeF 2 | MgF 2 | CaF 2 | SrF 2 | BaF 2 |
| Chlorides | BeCl 2 | MgCl 2 | CaCl 2 | SrCl 2 | BaCl 2 |
| Sulfates | BeSO 4 | MgSO 4 | CaSO 4 | SrSO 4 | BaSO 4 |
| Carbonates | BeCO 3 | MgCO 3 | CaCO 3 | SrCO 3 | BaCO 3 |
| Nitrates | Be (NO 3 ) 2 | Mg (NO 3 ) 2 | Ca (NO 3 ) 2 | Sr (NO 3 ) 2 | Ba (NO 3 ) 2 |
| Sulphides | BeS | MgS | CaS | SrS | Bas |
- Others
- In the Zintl phases , the associated anions form a remarkable lattice.
- Grignard compounds are magnesium compounds of the form R -Mg X . R stands for an organic radical and X is a halogen . They are used in organic synthesis .
-
Calcium carbide (Ca C 2 ) forms an ion lattice with Ca 2+ and (| C≡C |) 2− ions ( NaCl structure). The connection is required for three major procedures:
-
Ethyne production by hydrolysis :
- Ent desulphurisation of crude steel
- Azotation to calcium cyanamide
-
Ethyne production by hydrolysis :
- Calcium oxalate (Ca C 2 O 4 ) is the main component of kidney stones .
- Calcium cyanamide (Ca CN 2 ) is a fertilizer that is also used in other areas (e.g. for weed and pest control ).
- Strontium titanate (Sr Ti O 3 ) is traded as a gemstone under the name fabulite .
- Barium peroxide (Ba 2+ (OO) 2− ) used to play an important role in hydrogen peroxide synthesis:
Water hardness
Dissolved calcium and magnesium ions are mainly responsible for the hardness of the water . For example, the water-soluble calcium hydrogen carbonate (Ca (HCO 3 ) 2 ) changes into the poorly soluble compound calcium carbonate (CaCO 3 ), which is also known as " scale ":
The reverse reaction is prevented by the escape of carbon dioxide from the solution and the scale is deposited in cooking pots etc. Calcium hydrogen carbonate is therefore classified in the area of temporary water hardness.
Another property of alkaline earth metal ions, but especially Ca 2+ and Mg 2+ , is to form insoluble compounds with soap . Since soaps are salts from a chemical point of view , they consist of cations and anions . The anions are always higher fatty acids , and alkali metal ions are usually used as cations . The alkaline earth metal ions replace these and thus form insoluble compounds that are summarized under the term " lime soap ".
Occurrence
The alkaline earth metals are involved in the structure of the earth's crust, including the air and water envelope, as follows (data in% by weight):
- 2.7 · 10 −4 % beryllium
- 2.0 x 10 0 % magnesium
- 3.4 x 10 0 % calcium
- 3.6 · 10 −2 % strontium
- 4.0 · 10 −2 % barium
- 1.0 · 10 -10 % radium
The alkaline earth metals never occur naturally and are usually bound as silicate , carbonate or sulfate .
Beryllium-containing gemstones
Although beryllium is very rare, it is found in 30 different minerals . The best known include:
proof
The alkaline earth metals are detected primarily by spectral analysis based on the characteristic spectral lines . Wet chemical methods such as precipitation as carbonates , sulfates or hydroxides are now only used for demonstration purposes.
| ion | Flame color | Reaction with OH - | ... with CO 3 2− | ... with SO 4 2− | ... with C 2 O 4 2− | ... with CrO 4 2− |
|---|---|---|---|---|---|---|
| beryllium | no | Be (OH) 2 precipitates | BeCO 3 is soluble | BeSO 4 is soluble | BeC 2 O 4 precipitates | BeCrO 4 is soluble |
| magnesium | no | Mg (OH) 2 precipitates | MgCO 3 precipitates | MgSO 4 is soluble | MgC 2 O 4 is soluble | MgCrO 4 is soluble |
| Calcium | brick red | Ca (OH) 2 precipitates | CaCO 3 precipitates | CaSO 4 precipitates | CaC 2 O 4 precipitates | CaCrO 4 precipitates |
| strontium | intense red | Sr (OH) 2 precipitates | SrCO 3 precipitates | SrSO 4 precipitates | SrC 2 O 4 is soluble | SrCrO 4 precipitates |
| barium | yellow-green | Ba (OH) 2 is soluble | BaCO 3 precipitates | BaSO 4 precipitates | BaC 2 O 4 is soluble | BaCrO 4 precipitates |
| radium | carmine | Ra (OH) 2 is soluble | RaCO 3 fails | Raso 4 precipitates | RaC 2 O 4 precipitates | RaCrO 4 precipitates |
safety instructions
Only beryllium and magnesium are stable in air. The other elements of this main group must be stored under paraffin oil or inert gas . Storage under alcohol is only possible with beryllium, magnesium and calcium, since barium already splits off hydrogen from them and reacts to alcoholate .
Magnesium is highly flammable in finely divided form; Calcium, strontium and barium powders can self-ignite in air. Burning alkaline earth metals must never be extinguished with water!
The alkaline earth metals are strong reducing agents which are even able to release alkali metals from their compounds. These reactions are highly exothermic ; under certain circumstances it can even lead to an explosion .
Beryllium is a lung poison , although the mechanism of action is still largely unknown. Its compounds are also carcinogenic.
Barium compounds are highly toxic if they are readily soluble in water. 1 gram can be fatal.
Radium is extremely harmful to health due to its radioactivity, but until 1931 water mixed with radium was sold for drinking under the trade name Radithor . The number of those injured or perished who, like the steel magnate Eben Byers , consumed Radithor is unknown.
Web links
literature
- Hans Breuer: dtv-Atlas Chemie (Volume 1: General and Inorganic Chemistry) (2000), ISBN 3-423-03217-0 , pp. 94-113.
- Wolfgang Glöckner (Hrsg.): Handbook of experimental chemistry. tape 2 : alkali and alkaline earth metals, halogens. Aulis-Verl. Deubner, Hallbergmoos 1996, ISBN 3-7614-1816-7 .
Individual evidence
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1215.
- ↑ Duden Learn Attack GmbH: flame coloration
- ↑ a b c P. Häussinger, R. Glatthaar, W. Rhode, H. Kick, C. Benkmann, J. Weber, H.-J. Wunschel, V. Stenke, E. Leicht, H. Stenger: Noble Gases. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2006 ( doi : 10.1002 / 14356007.a17_485 ).
- ↑ Duden Learn Attack GmbH: alkaline earth metals



















