Strontium nitrate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
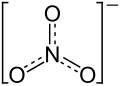
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Strontium nitrate | |||||||||||||||
| other names |
Nitric acid strontium |
|||||||||||||||
| Molecular formula | Sr (NO 3 ) 2 | |||||||||||||||
| Brief description |
colorless crystals that decompose in the heat |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 211.63 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.986 g cm −3 |
|||||||||||||||
| Melting point |
570 ° C |
|||||||||||||||
| boiling point |
decomposition |
|||||||||||||||
| solubility |
good in water (660 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−978.2 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Strontium nitrate , Sr (NO 3 ) 2 , is the strontium salt of nitric acid . It is used as an oxidizing agent in pyrotechnics and gives it the deep red flame color typical of strontium.
Manufacturing
Strontium nitrate is made by dissolving strontium carbonate in nitric acid.
properties
The anhydrous strontium nitrate crystallizes above 31.3 ° C., the tetrahydrate below this temperature from aqueous solution. Anhydrous strontium nitrate crystallizes in the cubic crystal system in the space group Pa 3 (space group no. 205) with the lattice parameter a = 778.13 pm . In the unit cell contains four formula units . The crystals are isotypic to barium nitrate . The tetrahydrate forms monoclinic crystals with the space group C 2 / c (No. 15) , the lattice parameters a = 1112 pm, b = 1417 pm, c = 634 pm, β = 123.75 ° and four formula units in the unit cell.
use
Strontium nitrate is u. a. used in pyrotechnics. Together with magnesium powder , at high temperature, favored by z. B. 5-amino-1 H -tetrazole or hexamethylenetetramine , briefly strontium (I) hydroxide (SrOH) is generated. This is a strong emitter in the red spectral range and acts as the sole color transmitter in bright and deeply saturated red pyrotechnic light packs .
Individual evidence
- ↑ Entry on strontium nitrate. In: Römpp Online . Georg Thieme Verlag, accessed on December 5, 2013.
- ↑ a b c d e f g Entry on strontium nitrate in the GESTIS substance database of the IFA , accessed on May 10, 2017(JavaScript required) .
- ↑ Strontium nitrate data sheet from Sigma-Aldrich , accessed on May 14, 2017 ( PDF ).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-16.
- ↑ JP MacMillan, JW Park, R. Gerstenberg, H. Wagner, K. Köhler, P. Wallbrecht: Strontium and Strontium Compounds in Ullmanns Encyclopedia of Industrial Chemistry, 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, doi : 10.1002 /14356007.a25_321 .
- ^ Gmelin's Handbook of Inorganic Chemistry: Strontium, System Number 29, Eighth Edition, Verlag Chemie GmbH, Berlin 1931, page 96.
- ↑ H. Nowotny, G. Heger: Structure refinement of strontium nitrate, Sr (NO 3 ) 2 , and barium nitrate, Ba (NO 3 ) 2 in Acta Cryst. 1983 , C39 , pp. 952-956 doi : 10.1107 / S0108270183006976
- ↑ B. Ribár, B. Matković and M. šljukić: The crystal structure of strontium nitrate tetrahydrate, Sr (NO 3 ) 2 · 4 H 2 O in Zeitschrift für Kristallographie 1972 , 135 , pp 137-144. doi : 10.1524 / zkri.1972.135.1-2.137
- ↑ Jesse J. Sabatini, Ernst-Christian Koch, Jay C. Poret, Jared D. Moretti, Seth M. Harbol: Red pyrotechnic flares - without chlorine! In: Angewandte Chemie . 127, 2015, pp. 11118–11120, doi : 10.1002 / anie.201505829 .


