Strontium carbonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
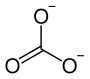
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Strontium carbonate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | SrCO 3 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 147.63 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.5 g cm −3 |
|||||||||||||||
| Melting point | ||||||||||||||||
| boiling point |
Decomposition from 1200 ° C |
|||||||||||||||
| solubility |
very bad in water (0.01 g l −1 at 18 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−1220.1 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Strontium carbonate , also outdated carbonic acid strontium , is the carbonate of strontium . It has the empirical formula SrCO 3 .
Occurrence
Strontium carbonate is found in nature as the mineral strontianite .
presentation
The preparation of strontium carbonate is effected by fusion of the mineral celestite (SrSO 4 ) and soda ( sodium carbonate ):
properties
Strontium carbonate is a fine, white powder that has properties similar to calcium carbonate . The salt serves as the starting material for the production of most strontium compounds.
Strontium carbonate does not dissolve in pure water , but in water containing carbon dioxide / carbonic acid . Here forms Strontiumhydrogencarbonat :
In mineral acids , soluble strontium salts are formed from strontium carbonate. The formation of carbonic acid is observed, which further breaks down into water and carbon dioxide.
At 1268 ° C under normal air pressure , the chemical compound splits into strontium oxide and carbon dioxide :
If the air pressure is increased to approx. 70 bar , strontium carbonate melts at 1497 ° C.
use
Strontium carbonate is used in ferrite magnets and serves as an absorber for X-rays in color television tubes . Electrolysis - Zinc can be cleaned with strontium carbonate. It is also used in glazes and, because of the red color of the flame, in pyrotechnics .
In the past, it was occasionally used in medicine to treat schizophrenia . However, it is no longer used because of the availability of more tolerable agents. Today the substance is only used in homeopathy under the name Strontium carbonicum , for example for osteoarthritis and cerebral sclerosis .
In organic synthesis, strontium carbonate can be used together with hydrogen and palladium for the selective reduction of double bonds .
swell
- ↑ a b c d e f Entry on strontium carbonate in the GESTIS substance database of the IFA , accessed on December 19, 2019 (JavaScript required)
- ^ Richard C. Ropp: Encyclopedia of the Alkaline Earth Compounds . Newnes, 2012, ISBN 0-444-59553-8 , pp. 367 ( limited preview in Google Book search).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-20.
- ↑ M. Binnewies et alii: Allgemeine und Anorganische Chemie . 2nd Edition. Spectrum, 2010, ISBN 3-8274-2533-6 , pp. 383 .
- ↑ Michael B Smith: Organic Synthesis. Academic Press, 2011, p. 431 ( limited preview in Google Book Search).



