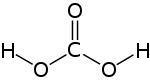
carbonic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | carbonic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | H 2 CO 3 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 62.03 g mol −1 | ||||||||||||||||||
| Physical state |
only exists in the presence of water in solution |
||||||||||||||||||
| pK s value |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Carbonic acid (H 2 CO 3 ) is an inorganic acid and the reaction product of its acid anhydride carbon dioxide (CO 2 ) with water . The salts of the two protonic acids are the carbonates and hydrogen carbonates . Their esters are also called carbonates or carbonic acid esters . The polyesters , which are known as polycarbonates , are of technical importance .
Compared to O 2 and N 2, the gas CO 2 is relatively soluble in water and reacts to a small extent (around 0.2%, depending on temperature and pressure ) to form carbonic acid:
The gas CO 2 is colloquially often imprecisely referred to as carbonic acid. In fact, in water chemistry, dissolved CO 2 is usually combined with the actual acid H 2 CO 3 as free carbonic acid . It stands in opposition to the sum of carbonate and hydrogen carbonate as the bound carbonic acid .
Carbon dioxide plays an important role in the acid-base balance of water as well as blood and body fluids.
The nature of carbonic acid as dissolved carbon dioxide was recognized by William Brownrigg in 1741 . However, Joseph Priestley is known for the invention of soda water (in his time as a priest in Leeds from 1767, where he had enough carbon dioxide from a nearby brewery).
Dissociation equilibrium
Dissolved carbon dioxide is in equilibrium with carbonic acid in aqueous solution:
- (1)
The Erlenmeyer rule describes the instability of molecules with two hydroxyl groups on the same carbon atom. Hence the equilibrium is very much on the side of the anhydride ; the proportion of the acid molecule in aqueous solution is only around 0.2%. This proportion is moderately dependent on the temperature. In organisms , the reaction is accelerated by the enzyme carbonic anhydrase . Carbonic acid is a biprotonic acid . It therefore releases its protons to water or other bases in two dissociation stages :
- (2)
The pK s value of the first acid constant can only be calculated. With temperature-dependent deviations, it is actually around 3.6. Carbonic acid is thus a medium-strength acid comparable to acetic acid (pK s 4.76) and formic acid (pK s 3.77). However, since it is difficult to determine the proportion of carbon dioxide according to equation (1), reactions (1) and (2) are summarized as (3):
- (3)
and result in the (almost always mentioned and experimentally determinable) value of approx. 6.5 for the pK s value. Free carbon dioxide is therefore a weak acid. The reaction product is the hydrogen carbonate ion HCO 3 - .
- (4)
The pK s value for the second acidity constant is 10.5. The reaction product is the carbonate ion CO 3 2− .
The concentrations of the three (actually four) carbonic acid species , i.e. the free carbonic acid (H 2 CO 3 and dissolved CO 2 ), the hydrogen carbonate and the carbonate as well as the oxonium ions are related to each other through the law of mass action . The concentration of oxonium ions is expressed in terms of pH . At a given pH, the proportion of the species is thus fixed.

pH indication water
- At pH 4, over 99% is present as a carbon dioxide / water mixture. (e.g. in mineral water )
- At a pH of 6.5, which is the same as the pK s of the first acid constant, there is also the same amount of hydrogen carbonate; the proportion of carbonate is still far below 1%.
- At around pH 7.5 in tap water, the hydrogen carbonate contained in it, in conjunction with little dissolved carbon dioxide, determines the acid balance. (human blood with pH 7.4 contains carbon dioxide and hydrogen carbonate in a ratio of 1:24)
- At around pH 8.3, the maximum proportion of hydrogen carbonate is around 98%; carbon dioxide and carbonate are each just under 1%. This is the typical pH of sodium or potassium hydrogen carbonate in water. Boiled drinking water also shows this pH, as dissolved carbon dioxide has been expelled.
- Drinking water that has been boiled down to almost dryness has a pH of up to 9, as a small proportion of hydrogen carbonate is converted into carbonate (see scale formation).
- At a pH equal to the pK s of the second acid constant of 10.5, the same amounts of hydrogen carbonate and carbonate and a negligibly small amount of carbon dioxide are present.
- At pH 12.5, the carbonate has a share of 99%, hydrogen carbonate is just under 1%. This is the typical pH of sodium or potassium carbonate in water.
These benchmarks reflect the relationships in the widely used bicarbonate buffer .
use
Carbon dioxide is used for countless production processes around the world, and it is probably best known to end consumers from soft drinks . Jacob Schweppe developed a process in the late 18th century by which water can be mixed with carbon dioxide. In the 19th century, carbon dioxide was added to mineral water to make it durable. See Use of Carbon Dioxide in Food Technology .
Carbonic acid as a pure substance
In the laboratory it has been possible to produce carbon dioxide (in the narrower sense) as a pure substance. However, this is currently of no practical importance. At low temperatures and in the absolute absence of water or metal ions (both strongly catalyze the decomposition reaction to carbon dioxide and water), the carbonic acid H 2 CO 3 can be represented as a water-clear, colorless liquid.
Derivatives of carbonic acid
In addition, organic derivatives of carbonic acid are known, such as various carbonic acid esters. They are easily accessible through the reaction of phosgene with alcohols. Polycarbonates are polyesters of carbonic acid . Another group of substances are the amides of carbonic acid. Their parent compound is urea , a diamide of carbonic acid; the urethanes (from urea , urea) are mentioned as an example . They are substituted esters of the monoamide of carbonic acid, of carbamic acid ; these are the main compounds of extremely important plastics, the polyurethanes .
Aggressive carbon dioxide and relatives
Another group of trivial names , which do not denote chemically different species but rather proportions , comes from the field of water chemistry for calcareous waters. It should be noted that the following terms each relate to proportions of so-called free carbonic acid , in which no distinction is made between carbon dioxide and carbonic acid in the narrower sense.
According to the lime-carbonic acid balance , the concentrations of calcium and carbonic acid are dependent on one another. A distinction is made between the amount of the associated carbon dioxide and the amount of excess and (lime) aggressive carbon dioxide . Corresponding acid keeps the pH value just so low in the quantitative equilibrium of the carbonic acid species that the dependent concentration of the carbonate multiplied by that of the calcium does not just exceed the solubility product of the calcium carbonate. Any free carbon dioxide that is also present is considered to be excess. A part of this could bring more lime into solution, so it is (lime) aggressive ; the rest of the excess would then be required as additional associated carbonic acid.
With increasing values for the carbonate hardness, the proportion of the associated free carbonic acid increases disproportionately. For example, this value is 1.83 mg / l CO 2 at 5.1 ° dH and 11.67 mg / l CO 2 at 10.2 ° dH . This leads to a mixed water problem when mixing water . Mixing water with different carbonate hardness results in mixed water with aggressive carbonic acid, even if the source water was in the lime-carbonic acid balance.
The mathematical relationships are summarized in Tillmans ' equation, with which the associated “free carbonic acid” can be calculated for each calcium content. The short version of this equation follows:
The elements of the equation mean:
- = Concentration of the corresponding free carbon dioxide (CO 2 ) to be calculated in mol / kg
- = Tillmans constant
- = Square of the concentration of hydrogen carbonates (HCO 3 ) in mol / kg
- = Concentration of calcium in mol / kg
For more details, see Tillmans equation .
Precise knowledge of this equilibrium and its setting speed is of great importance for the treatment and decarbonization of water. For example, when treating drinking water, the raw water is passed over half-burnt dolomite (calcium magnesium carbonate, CaMg (CO 3 ) 2 ) so that it does not contain any excess "free carbonic acid", as iron or other metals would react with it and thus corrode steel pipes, for example. These reactions are also in equilibrium with the corresponding carbonates, depending on the concentration. Therefore one speaks then z. B. of "iron-aggressive carbon dioxide". Dolomite is used because in the presence of magnesium ions the speed at which Tillmans equilibrium is established is considerably accelerated, which would take too long with pure calcium carbonate.
For some user groups, e.g. B. in fishing, the quantity terms mentioned here are often misunderstood as if z. B. the "aggressive carbon dioxide" would be particularly harmful, for example for the fish. But since fish are not made of lime, the aggressive carbonic acid is not directed against them in any other way than the rest of the carbonic acid. Rather, it is the total dissolved CO 2 concentration that is decisive for the breathing of the fish, and the pH value of the water is the only decisive factor for any acidic corrosion. The "associated carbonic acid" is misunderstood there as if it were bound to the hydrogen carbonate in a special way and therefore could not be expelled by water ventilation or consumed by algae through photosynthesis. In fact, all of the “free carbon dioxide” is available to both processes, so that the pH value increases and the quantity equilibrium shifts towards more carbonate, and ultimately the lime solubility product is exceeded, i.e. lime precipitation . See also lime-carbonic acid balance .
literature
- Ulrich Kölle: All about carbon dioxide. In: Chemkon. 10, No. 2, 2003, pp. 66-68.
- Th. Loerting, Chr. Tautermann, R. T. Kroemer: On the Surprising Kinetic Stability of Carbonic Acid. In: Angew. Chem. Int. Ed. 39, No. 5, 2000, pp. 891-894.
German: Th. Loerting, Chr. Tautermann, R. T. Kroemer: To the surprising kinetic stability of carbonic acid. In: Angew. Chem. 112, 2000, pp. 919-922 ( doi : 10.1002 / (SICI) 1521-3757 (20000303) 112: 5 <919 :: AID-ANGE919> 3.0.CO; 2-Y ). - Kurt Bauer: On the importance of carbon dioxide in carp ponds. In: Österreichs Fischerei 44, 1991, pp. 49-64.
- Julius Pia : Carbon dioxide and lime - an introduction to understanding their behavior in inland waters. In: The inland waters. Vol. XIII, Schweizerbart, Stuttgart 1933, ISBN 978-3-510-40713-2 .
- HE Hömig: Physicochemical basics of feedwater chemistry. 2nd edition, Vulkan-Verlag, Essen 1963, chap. 2.25-2.30.
Individual evidence
- ↑ entry to carbonic acid in the CosIng database of the European Commission, accessed on 4 March 2020th
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- ↑ K. Adamczyk, M. Prémont-Schwarz, D. Pines, E. Pines and ETJ Nibbering: Real-Time Observation of Carbonic Acid Formation in Aqueous Solution. In: Science . 2009, 326, pp. 1690-1694, doi : 10.1126 / science.1180060 . - C. Ho and JM Sturtevant The Kinetics of the Hydration of Carbon Dioxide at 25 . In: J. Biol. Chem. 238 , 3499-3501 (1963) PDF .
- ^ DH Ripin, DA Evans: pKa's of Inorganic and Oxo-Acids ( English , PDF) Retrieved July 15, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Report: uni-protocol.de . - T. Loerting, C. Tautermann, RT Kroemer, I. Kohl, A. Hallbrucker, E. Mayer, KR Liedl: in Angewandte Chemie 2000 , 112 , 919-922. On the surprising kinetic stability of carbonic acid (H 2 CO 3 ) , doi : 10.1002 / (SICI) 1521-3757 (20000303) 112: 5 <919 :: AID-ANGE919> 3.0.CO; 2-Y .
- ↑ On the importance of carbon dioxide in carp ponds . Partial print of the original.