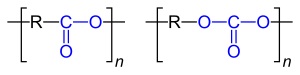polyester
| General structure of polyesters |
| Left: Repeat unit for polyesters made from lactones . These polyesters are carboxylic acid esters . Right: Repeat unit for polycarbonates , these are carbonic acid esters (carbonates). They are often condensates of dihydroxy compounds and phosgene [Cl− (CO) −Cl]. The two different ester functions are marked in blue . R stands for the “remainder” of the compounds used for the synthesis. |
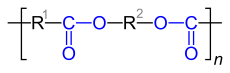
| Repeating units of polyesters made from dicarboxylic acids and dihydroxy compounds. R 1 stands for the “remainder” of the dicarboxylic acid used, R 2 for the “remainder” of the dihydroxy compound used. |
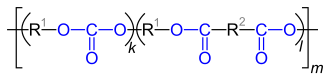
| Repeating units in copolymers of carbonates and carboxylic acid esters (polyester carbonates). R 2 stands for the “remainder” of the dicarboxylic acid used, R 1 for the “remainder” of the dihydroxy compound used. |
Polyesters are polymers with ester functions - [- CO – O -] - in their main chain. Although polyesters also occur in nature, today polyester is more a large family of synthetic polymers ( plastics ), which include the widely used polycarbonates (PC) and, above all, the technically important thermoplastic polyethylene terephthalate (PET). Another form are unsaturated polyester resins (UP resins), which harden to form thermosetting plastics and are used as an inexpensive matrix resin in the field of fiber-reinforced plastics . In addition, aromatic polyesters can be arranged to form liquid-crystalline polymer chains, which results in the property profile of a high-performance plastic.
history
Natural polyesters have been known since around 1830. The first synthetic polyester glycerol phthalate was used as an impregnation agent during World War I ; Alkyd resins came onto the market as Glyptal at General Electric in the 1920s. They were developed as textile fibers in the Wallace Hume Carothers group at DuPont , but they were not yet heat-resistant, which was only achieved by John Rex Whinfield in England in the early 1940s. The first such fiber was produced as Terylene at Imperial Chemical Industries soon after World War II .
Applications

- Polyester fibers (PES) for textiles , nonwovens and microfibers
- PET bottles
- Foils , e.g. B. copyable film for overhead projectors , base material for flexible circuit boards ( biaxially oriented polyester films ), dielectric for capacitors
- photographic films (next to cellulose triacetate , polyester is the most important carrier material)
- unsaturated polyester resins for casting resins and fiber composite materials
- as alkyd resins for paints
- Polyester polyols are used as raw material in the reaction of diisocyanates to form polyurethanes .
Abbreviations
- PBT: polybutylene terephthalate , a polymer of terephthalic acid
- PET: polyethylene terephthalate , a polymer of terephthalic acid
- PLA: polylactide , the biodegradable polymer of lactic acid . The polymer of D - (-) - lactic acid is also abbreviated as PDLA, that of L - (+) - lactic acid with PLLA. PDLLA is the poly-D, L-lactic acid.
- PTT: polytrimethylene terephthalate
- PEN: polyethylene naphthalate
- PC: polycarbonate , an ester of carbonic acid
- PEC: polyester carbonate, as well as carboxylic acid esters and esters of carbonic acid
- PAR: polyarylates , aromatic polyesters
- UP: unsaturated polyester resin
synthesis
The polyester synthesis generally takes place in a polycondensation reaction or by ring-opening polymerization .
Azeotropic esterification
The azeotropic esterification is a classic (laboratory) method in which an alcohol and a carboxylic acid react to form a carboxylic acid ester . In order to produce a polymer from diol and dicarboxylic acid, the water formed during the reaction has to be constantly removed by azeotropic distillation in order to shift the chemical equilibrium to the ester side. The reaction is catalyzed by titanium or tin (IV) alcoholates at 180–240 ° C. You can add about 2% xylene as a water tractor. By suitable choice of the starting materials, it is possible to produce polyesters containing hydroxyl groups. The degree of esterification is usually> 95%, determined by an accompanying acid number determination.
Transesterification
During transesterification , a diol in the melt is reacted with a dicarboxylic acid ester (e.g. dimethyl terephthalate ) at the catalyst contact. This method is used to manufacture the bulk plastics polybutylene terephthalate (PBT) and polyethylene terephthalate (PET).
Carboxylic acid chloride method
Instead of carboxylic acids, carboxylic acid chlorides are used. The polycondensation takes place with elimination of hydrogen chloride (HCl) instead of water. This acylation method can be carried out in solvents , as an interphase or as a melt reaction .
- Silyl method
- In this variant of the hydrochloric acid method, the carboxylic acid chloride is reacted with the trimethylsilyl ether of the alcohol component; trimethylsilyl chloride is split off.
Acetate method (transesterification)
In this method, which is only suitable for phenolic hydroxyl groups, the acid reacts with the alcohol component that has already been esterified with acetic acid. During condensation, acetic acid is formed, which cannot be removed as easily as water or hydrochloric acid, which lowers the pH value and often leads to acidic side reactions.
- Silyl acetate method
- In this variant of the acetate method, it is not the carboxylic acid but its trimethylsilyl ester that is used. The result is the trimethylsilyl acetate, which is not acidic.
Ring-opening polymerization
In ring-opening polymerization , aliphatic polyesters can be produced from lactones via anionic, cationic, coordinative chain polymerization or enzyme-based without a condensation reaction under very mild conditions .
Trade names
Fibers, fabrics and Fleecestoffe ( fleece ) of polyester are marketed under various trade names sold:
- Polarguard
- Thermolite
- Trevira
- Dacron
- Diols
- Sorona , a PTT from DuPont, which is based on bio-based 1,3-propoanediol (PDO).
- Terylene
- Vestan
- Grisuten
- Tritan , a copolyester from Eastman Chemical , which - as far as is known - is derived from the alcohols ethylene glycol and 2,2,4,4-tetramethyl-1,3-cyclobutanediol and the acid terephthalic acid. He is z. B. used for drinking bottles and is considered to be less problematic than conventional PET.
Polyester varnish
Polyester lacquers are solutions of unsaturated polyesters (e.g. maleic acid glycol ester) in a monomer (e.g. styrene) to which organic peroxides are added as a reaction accelerator. Polyester paints are used as solvent-free or low-solvent paints. The films obtained have a high resistance to weathering and chemicals. The main area of application of polyester lacquers is the production of colorless and pigmented wood lacquers as well as fillers for wood and iron. In addition, polyester is used in the manufacture and application of powder coatings.
Web links
Individual evidence
- ↑ Joachim Buddrus: Fundamentals of organic chemistry. Walter de Gruyter Verlag, Berlin, 4th edition, 2011, p. 901, ISBN 978-3-11-024894-4 .
- ↑ Charles Carraher: polyester, Chemistry explained ' .
- ^ Article Whinfield, Lexicon of important chemists , Harri Deutsch, 1989.
- ^ Oskar Nuyken, Heidi Samarian, Ilse Wurdack: Polymers in medical technology. In: ChemgaPedia. ChemgaPedia from Wiley Information Services GmbH, p. 8 , accessed on August 30, 2016 .
- ↑ Indra K. Varma, Ann-Christine Albertsson, Ritimoni Rajkhowa, Rajiv K. Srivastava: Enzyme catalyzed synthesis of polyesters . In: Progress in Polymer Science . tape 30 , no. 10 , p. 949-981 , doi : 10.1016 / j.progpolymsci.2005.06.010 ( elsevier.com [accessed January 25, 2018]).
- ↑ Fachuni Chemie Berlin - plastic table (polycondensates / polyester blend) .
- ↑ Reference to Grisuten as a trademark of the GDR .
- ↑ Paul Dornath, Skip Rochefort: Analysis of "BPA-free" Tritan ™ copolyester Under High Stress Conditions. (PDF) (No longer available online.) Oregon State University's Subsurface Biosphere Initiative, archived from the original March 3, 2016 ; accessed on August 30, 2016 (English). Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Andreas Winterer: Tritan : Drinking bottle made of plastic without BPA - harmless? Utopia GmbH Munich, June 15, 2016, accessed on August 30, 2016 .
- ↑ Tritan drinking water bottles. Öko-Treff im Lichtental, accessed on August 30, 2016 .
- ^ Encyclopedia entry Lacke , website of the journal 'Spektrum der Wissenschaft'.