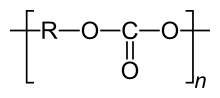
Polycarbonates
Polycarbonate ( abbreviated PC ) are thermoplastic plastics . They are formally polyesters of carbonic acid .
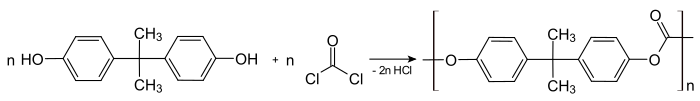
Polycarbonates can be produced by polycondensation of phosgene with diols . They are of practical importance for the synthesis of aromatic bishydroxy compounds, such as bisphenol A , for example . As an alternative to the dangerous phosgene, transesterification with carbonic acid diesters can also take place. The recycling code for polycarbonates is 07 (other plastics).
history
Although Alfred Einhorn discovered aromatic polycarbonates as early as 1898 , the first industrially relevant polycarbonate was not developed until 1953 by Hermann Schnell at Bayer AG . This was based on 2,2-bis (4-hydroxyphenyl) propane ( bisphenol A ). Bayer began large-scale production under the trade name Makrolon in 1958 . Bayer later expanded this brand name to include other polycarbonates. 1973 General Electric followed with large-scale production under the trade name Lexan (today it belongs to the manufacturer SABIC ).
synthesis
The most common polycarbonates are those which use bisphenol A as the dihydroxy component and phosgene .
The production takes place via interfacial condensation. The aqueous phase consists of sodium hydroxide solution in which bisphenol A dissolves as the sodium salt. The gaseous phosgene is introduced into the organic phase of, for example, dichloromethane . Tertiary amines act as catalysts ; the reaction starts already at room temperature. Hydrogen chloride is immediately converted into sodium chloride with the sodium hydroxide solution.
Alternatively, it can be produced via transesterification with diphenyl carbonate . In this melt condensation, the reaction takes place under protective gas at low pressure, the reaction product phenol is removed from the reaction mass by the negative pressure:
The reaction is started at 180 to 220 ° C, bases are used as catalysts. The polycondensation is completed at up to 300 ° C and underpressure.
variants
Instead of bisphenol A, the following hydroxy compounds are also used:

Bisphenol S.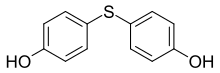
Dihydroxydiphenyl sulfide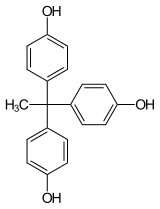
1,1,1-tris (4-hydroxyphenyl) ethane
(THPE)
Tetramethyl bisphenol A.
1,1-bis (4-hydroxyphenyl) -
3,3,5-trimethylcyclohexane
(BPTMC)
By using mixtures of the above components properties of the resulting polycarbonate z. T. can be varied continuously. Cocondensates made from bisphenol A and BPTMC lead to highly transparent, heat-resistant plastics. THPE allows the incorporation of chain branches that have a positive effect on the structural viscosity during processing of the material (for example during extrusion ). Dihydroxydiphenyl sulfide leads to a high refractive index of the plastic, which is advantageous for the production of optical lenses.
Another synthesis route is used for the thermoset polyallyl diglycol carbonate , where the monomer is already a carbonate and is polymerized by free radicals.
properties
| property | unit | value |
|---|---|---|
| density | g cm −3 | 1.20 |
| Modulus of elasticity | MPa | 2400 |
| tensile strenght | MPa | 65 |
| Elongation at break | % | 120 |
| Rockwell hardness (according to ISO 2039) | - | R122 |
| Heat resistance HDT A (ISO 75) | ° C | 125 |
| Notched impact strength (ISO 7391 / i. A.,
ISO 179 / IeA) |
kJ m −2 | 60 |
Polycarbonates usually have a crystallite content of less than 5% and are therefore considered amorphous . They are characterized by high strength , impact strength , rigidity and hardness . In addition, polycarbonates are good insulators against electrical voltage.
Polycarbonates are resistant to water , many mineral acids and aqueous solutions of neutral salts and oxidizing agents . Even some non-polar organic solvents such as hydrocarbons and many oils and fats do not attack polycarbonates. In contrast, polycarbonates are not resistant to some chlorinated hydrocarbons, such as dichloromethane . Also, alkaline aqueous solutions, amines and ammonia , and some organic solvents attack polycarbonates.
Polycarbonates are flammable, but the flame goes out when the ignition source is removed. Polycarbonate meets the requirements of fire class B2 according to DIN 4102. In layers between one and six millimeters thick, it is classified in fire class B1, "hardly inflammable", for indoor applications. The requirements for the fire behavior of PC vehicle windows in accordance with approval guidelines such as TA29 (national), ECE43 or ANSI Z26.1 (USA) are also met.
The water-clear plastic is characterized by glass-like light transmittance (88% at three millimeters thick according to DIN 5036-1) and refractive indices (1.59 according to ISO 489-A).
Polycarbonate is sensitive to UV light in the wavelength range around 340 nm. Irradiation with light of this wavelength, etc. a. When used outdoors, without a protective coating, it leads to breaks and rearrangements in the polymer molecule, which over time cause the material to become brittle and yellow.
The maximum usage temperature is 125 ° C, briefly up to 135 ° C. The glass transition temperature is 148 ° C. Like all amorphous plastics, polycarbonate has no melting point.
Applications
Polycarbonates are transparent and colorless. However, they can be colored.
Polycarbonate is relatively expensive. It is therefore almost only used where other plastics are too soft, too fragile, too sensitive to scratches, too little dimensionally stable or not sufficiently transparent. In addition, polycarbonate is used as a transparent plastic, as is polymethyl methacrylate (PMMA) or styrene-acrylonitrile (SAN) as a glass alternative. Compared to brittle glass, polycarbonate is lighter and significantly more impact-resistant. In addition, there is no risk of splinters forming at moderate impact energies or speeds.
The applicability as a transparent glass alternative can be limited by the lower abrasion resistance of the polycarbonate. In the Taber test according to ASTM D1044 (DIN 52347 or ISO 15082 for plastic glazing), the plastic only achieves ∆Haze values of around 30% after 100 cycles. B. drifting sand clearly a. This shortcoming can be compensated for with coatings based on polysiloxanes, polyepoxides or polyurethane acrylates. Even polysiloxane lacquer layers of five to eight micrometers thick can equip polycarbonate semi-finished products or workpieces with a glass-like hard surface (∆ Haze after 100 cycles <4%) without impairing the ability to be hot-formed.
The chemical and UV resistance of the material can also be significantly increased by using suitable varnishes. Specialized coatings can also equip polycarbonates with self-cleaning or water-repellent surfaces. The discharge of static electricity is also possible, which allows the polycarbonate panes to be used, for example, as machine covers in explosion-protected areas.
In combination with transparent thermoplastic polyurethanes (TPU), light, UV-resistant composite safety glazing can be made from polycarbonate, which also performs well in terms of the important Head Injury Criterion (HIC) according to DIN 52310. The limit value HIC <1,000 required by TA29 or ECE 43 (guideline for vehicle glazing) is undercut by these laminated safety windows.
Polycarbonates are used, among other things, for the production of:
- CDs , DVDs, and Blu-ray Discs
- Eyeglass lenses and optical lenses
- Diffuser lenses from car headlights
- Jet aircraft windows
- Suitcases
- burglar-resistant glazing
- Underwater housings for cameras
- Back covers of cell phones (mostly smartphones ) and tablet computers
- Glazing of winter gardens and greenhouses
- Cladding of avant-garde buildings
- Solar panels
- Protective helmets and visors
- Camping dishes
- because of good biocompatibility with a large number of single-use medical products
- Microfibers with the electrostatic spray method
The world consumption of polycarbonate in 2009 was around 3 million tons, which corresponds to a value of around € 6 billion.
processing
Polycarbonates can be processed with all of the usual methods for thermoplastics . In injection molding , a high injection pressure is required because of the high viscosity of the melt. The processing temperatures are between 280 and 320 ° C and between 240 and 280 ° C for extrusion . Before processing, however, the residual moisture must be brought to below 0.01 percent by drying (4 to 24 hours at 120 ° C). The molding shrinkage of polycarbonate is between 0.6 and 0.8 percent. Polycarbonate shows almost no post-shrinkage. It can be bonded with solvents such as dichloromethane and reaction resin adhesives and can be ultrasonically and high-frequency welded.
safety instructions
According to American and Japanese studies, certain polycarbonates, for the production of which the monomer bisphenol A was used, can be released again when heated. Bisphenol A is suspected of causing considerable damage to health . In the EU, therefore, the use of polycarbonate containing bisphenol A, for example as a material for baby bottles, is prohibited.
recycling

The recycling code for polycarbonates is 07.
Trivia
The 180 windows of the Brussels Atomium have been made of coated polycarbonate since the restoration in 2006.
The roof of the Athens Olympic Stadium consists of around 25,000 square meters of polycarbonate sheets, which were previously provided with a UV-resistant coating in a North German company in Geesthacht, Schleswig-Holstein.
The Lamborghini Gallardo Superleggera has u. a. via a rear window and an engine compartment cover made of abrasion-resistant coated, UV-resistant polycarbonate. The components were created by hot forming and CNC milling of polycarbonate semi-finished products. The reason for the choice of material was, among other things, the lower weight of the material compared to glass: the lower the center of gravity of a vehicle, the better it is on the road. Replacing heavy glass with lighter, transparent plastics not only helps to save weight and thus fuel, but also to lower the vehicle's center of gravity. In civil automobiles, according to the car glass standard ECE 43R, only ΔHaze values below ten percent (500 U) are required behind the B-pillar (outside area). These values are well achieved by polysiloxane-coated polycarbonate.
The Grevenbroich artist Matthias Hintz makes sculptures out of polycarbonate. To do this, he assembles up to 20,000 CDs with a hot air blower.
Web links
- MATERIAL ARCHIVE: Polycarbonate - Extensive material information and pictures
Individual evidence
- ↑ a b c Entry on polycarbonates. In: Römpp Online . Georg Thieme Verlag, accessed on June 12, 2014.
- ↑ Hans Domininghaus (Ed.): The plastics and their properties . 6th, revised and expanded edition, Springer-Verlag Berlin / Heidelberg 2005, ISBN 3-540-21410-0 , p. 1019.
- ↑ a b Hans Domininghaus, Peter Elsner, Peter Eyerer, Thomas Hirth: plastics. Properties and uses . Ed .: Peter Elsner, Peter Eyerer, Thomas Hirth. 8th edition. Springer-Verlag, Heidelberg 2012.
- ^ A b Wolfgang Kaiser : Synthetic chemistry for engineers. 3. Edition. Carl Hanser, Munich 2011, p. 331 ff.
- ↑ Gerd Brammer: Coating provides a perspective. In: Kunststoffe, issue 3/2005, pp. 78–81.
- ↑ Korinna Brammer et al .: (glass) clear thing. In: Kunststoffe, issue 8/2006, pp. 84–87.
- ↑ De Zeen: Polycarbonates. Retrieved March 27, 2020 .
- ↑ Walter Loy: Chemical fibers for technical textile products. 2nd, fundamental revised and expanded edition. Deutscher Fachverlag, Frankfurt am Main 2008, ISBN 978-3-86641-197-5 , p. 258.
- ^ Dietrich Braun: Small history of plastics, 2nd edition. Carl Hanser Verlag, Munich 2017, ISBN 978-3-446-44832-2 , p. 290.
- ↑ Polycarbonate Plastics and Bisphenol A Release .
- ↑ EU coordination of German nature conservation ring (DNR): EU-wide ban on bisphenol A in baby bottles from June 2011. Report of November 26, 2010.
- ↑ European Commission: Bisphenol A: EU ban on baby bottles comes into force tomorrow . Press release from May 31, 2011.
- ↑ Stefan Albus: Polycarbonate - the marble of tomorrow? In: K-Zeitung, Issue 16/2012, pp. 25-26.