Polycondensation
Polycondensation is a multiple condensation reaction that converts monomers into polymers ( plastics ). In order for a monomer to be able to take part in the reaction, it must have at least two functional groups that are particularly reactive (e.g. –OH, –COOH, –NH 2 , –CHO…). As a rule, bifunctional monomers of various types (e.g. diols and dicarboxylic acids , which condense to form esters ) are reacted with one another. Polycondensation takes place in stages (stage growth reaction ) via stable but still reactive intermediates ( oligomers ). The oligomers react with one another and ultimately form a macromolecule. The products are called polycondensates . In addition to plastics, there are also a number of natural polymers, e.g. B. Poly silicas , which are formed by polycondensation.
In contrast to polymer production by chain polymerization or polyaddition , at least one by-product is released during polycondensation. These by-products (e.g. water, ammonia, lower alcohols, hydrogen chloride) must be removed continuously, otherwise the polycondensation will stop for thermodynamic reasons if the degree of polymerization is too low .
In contrast to chain polymerization, which takes place via chain growth, the reaction conversion in polycondensation must be at least 99% in order to obtain a polycondensate with a high molar mass. Furthermore, the ratio of the amounts of the respective monomer used must be adapted as precisely as possible to the stoichiometric ratio given by the reaction , otherwise one arrives at a point at which all oligomers have the same active ends and can no longer react with one another ( Carothers equation ).
Historical information
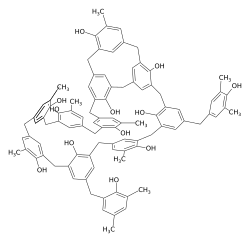 Structure of bakelite
Structure of bakelite
The first polycondensation was achieved by the German chemist and Nobel Prize winner Adolf von Baeyer in 1872. He described the polycondensation reaction of phenol and formaldehyde to form Bakelite and thus laid the basis for today's polymer chemistry . Bakelite was first produced on a large scale in 1909 by Leo Hendrik Baekeland and has been used in many areas for decades. It is still made today.
application
Polycondensation is an important process in polymer chemistry , with which numerous important plastics such as phenoplast (e.g. Bakelite ), polyester and polyamides are produced. Polycondensation is also of great importance in the production of adhesives , such as phenol-formaldehyde adhesives , and in the production of brake linings for motor vehicles .
Examples
Phenoplast
The reaction of phenol with an aldehyde produces a first intermediate product with the help of a catalyst .
This intermediate product reacts repeatedly with formaldehyde and phenol to form a macromolecule , splitting off water :
This is a condensation reaction , since water is repeatedly split off in the ongoing polymer formation. There is a copolymerization and a spatial crosslinking and one speaks of polycondensation. This creates a phenoplast (also called phenolic resin, phenol-formaldehyde condensate or Bakelite).
polyester
The reaction of carboxylic acids (here terephthalic acid ) with diols (compounds with two alcohol groups , here ethanediol ) forms a polyester (e.g. polyethylene terephthalate (PET)) with elimination of water . If this reaction takes place in several stages, including the reaction product as the starting material , it is a polycondensation.
If, for example, glycerine is used as a starting material instead of ethanediol , a spatial crosslinking occurs and a thermoset is formed .
Polyamides
Diamines react with dicarboxylic acids by polycondensation to form polyamides (e.g. nylon ):
If a mixture of adipic acid and hexamethylenediamine is heated, polycondensation takes place to form 6,6 nylon .
Technical procedures
- Solution polycondensation
- Melt polycondensation
- Interfacial polycondensation
- Solid phase polycondensation
- Precipitation Polycondensation
See also
Individual evidence
- ^ MD Lechner, K. Gehrke and EH Nordmeier: Makromolekulare Chemie , 4th edition, Birkhäuser Verlag, 2010, pp. 119-136, ISBN 978-3-7643-8890-4 .
- ^ Adalbert Wollrab: Organic chemistry . Springer-Verlag, 1999, ISBN 3-540-43998-6 , pp. 527 .



