Carothers equation
The Carothers equation describes the relationship between the degree of polymerization and the degree of conversion in a step growth reaction . It is named after Wallace Hume Carothers . There are several variants, for linear A – B systems, linear A – A / B – B systems and non-linear step growth reactions. In linear AB systems, there is a monomer in which the monomer carries two functional groups , such as B. at HO-R-COOH. In linear A – A / B – B systems, there are 2 monomers, each with one of the functional groups at both ends, such as B. HOOC – Ph – COOH and HO– (CH 2 ) 2 –OH, which can react to form polyethylene terephthalate . In non-linear systems, for. For example, in addition to A – B monomers, there are also trifunctional monomers, which leads to crosslinking of the product.
Linear step growth responses
AB systems
If the number of monomers originally present and the number of molecules still present at the time is ( includes all degrees of polymerization: monomers, oligomers and polymers), the conversion is obtained
(1)
p is the probability that one of the groups responded. With a turnover of , the probability that a group has responded is 50%.
The degree of polymerization - the average length of the chains - can be expressed as the fraction of the number of monomers initially present by the molecules still present at time t:
(2)
By transforming Eq. 1
and insert in Eq. 2 one obtains the Carothers equation for AB systems
AA / BB systems
For AA / BB systems it must also be noted that the system cannot be composed stoichiometrically, i. H. different monomer ratio can occur. That's why you define a parameter :
The parameter is always defined so that there is, i.e. there is more BB in the system than AA.
This gives you as
At the time , AA molecules have already been converted into turnover . For , the sum of converted AA and BB therefore applies .
The amount of unreacted monomers is accordingly
In the same way as above, substituting the following expression for
what the Carothers equation for AA / BB-systems corresponding
Nonlinear step growth reactions
AB systems
If trifunctional monomers are added to the monomer , a network is formed.
In order to be able to calculate the degree of polymerization, an average functionality of the monomers is defined
Here, the number of functional groups on the molecule i and the number of monomer molecules.
In the case of monomer molecules, functional groups are present overall .
After a time t, groups have reacted, since two end groups have to react for a bond. This resulted in the formation of molecules. So the probability of a reaction is
(3)
Transforming Eq. 3 results
and after insertion in Eq. 2 one obtains a Carothers equation for nonlinear systems
Equation 3 can also be transformed
(4)
When the degree of polymerization approaches infinity, gelation occurs and in Eq. 4 goes the expression
The following applies to the turnover where the mixture begins to gel:
From this relationship it can be seen that a high degree of polymerization can be achieved even with significantly lower conversions than in the other cases.
This equation only applies if the mixture is composed stoichiometrically (same number of A and B groups).
Graphic representation of conversion and degree of polymerization
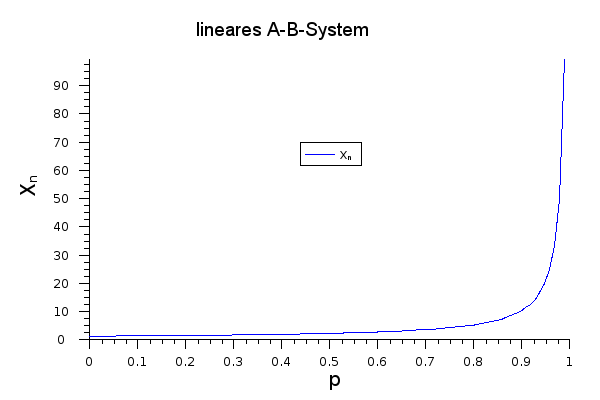
The meaning of the Carothers equation can be seen if the degree of polymerization is plotted against the conversion p:
Only at very high conversions does the degree of polymerization reach appreciably high values. At p = 0.5 it is just 2, and a value of 10,000 is only achieved at a conversion rate of p = 0.9999.
In the same way, r has a significant influence on the degree of polymerization:
Even small deviations of r from the ideal value 1 mean a significantly lower degree of polymerization.
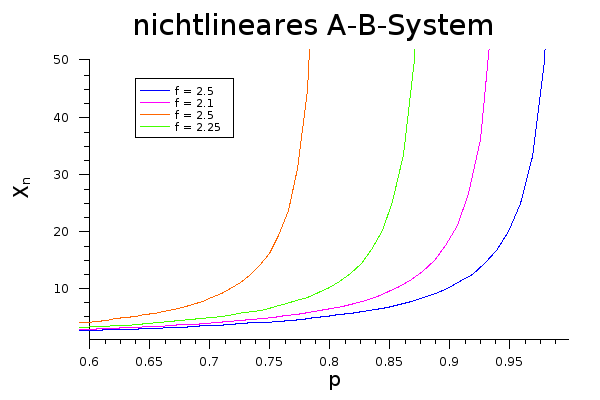
With the addition of crosslinkers, on the other hand, the conversion increases sharply even at a lower level:
Individual evidence
- ^ Polymers: Chemistry and Physics of Modern Material, by John McKenzie, Grant Cowie . books.google.de. Retrieved May 23, 2009.
Web links
- Macromolecules at TU Chemnitz, page 25 (PDF file; 505 kB)