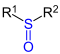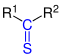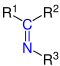Functional group
In organic chemistry , a functional group (also a characteristic group ) is the group of atoms in a compound that significantly determines the material properties and the reaction behavior of the compound. A compound can also carry several functional groups with different properties. Compounds that have the same functional groups but different alkyl or aryl radicals are grouped into substance classes due to their similar properties .
Functional groups are divided into functional groups with heteroatoms (mostly O, N, S, P, halogens) and those without heteroatoms (e.g. C = C double bonds , C≡C triple bonds or aromatics ) based on the atoms involved . Even if the latter are sometimes not viewed as “fully-fledged” functional groups, but only as structural building blocks, they still show a special and typical reaction behavior that justifies the designation functional group and the respective grouping into substance classes.
In the case of inorganic or organometallic compounds, simple alkyl or aryl groups are also regarded as functional groups. All organic side chains and functional groups are then summarized under the general name organyl group .
There are abbreviations for functional groups to simplify the writing in structural formulas and texts, which are listed in the list of abbreviations in organic chemistry .
properties
According to the definition, a functional group usually has a considerable influence on the chemical and physical properties of the starting compound it contains. The type of influence is demonstrated using the example of the alkane n -butane and three of its structural derivatives with the same number of carbon atoms:
| Structural descendant with the same number of carbon atoms |
connection | Semi-structural formula | properties |
|---|---|---|---|
| Output compound | n -Butane | H 3 C-CH 2 -CH 2 -CH 3 | gaseous |
| Carboxy compound | Butanoic acid , also butyric acid | H 3 C-CH 2 -CH 2 -COOH | liquid, foul smelling, reacts sour |
| Amino compound | Butan-2-amine , also 2-butylamine | H 3 C-CH (NH 2 ) -CH 2 -CH 3 | liquid, foul smelling, has a basic reaction |
| Carboxy and amino compound | 4-aminobutyric acid, also 4-aminobutyric acid or γ-aminobutyric acid (GABA) |
H 2 N-CH 2 -CH 2 -CH 2 -COOH | solid, exists
in aqueous solution as a zwitterion H 3 N + –CH 2 –CH 2 –CH 2 –COO - |
Different functional groups and their nomenclature
Basically, the substitutive nomenclature , which is described below, should be used to name substances. According to this nomenclature, the functional group is either a prefix ( prefix ) or as a suffix ( suffix included) on behalf of the total composition.
See also: nomenclature
Functional groups (in order of decreasing priority)
Either prefixes or suffixes are used to designate these groups in a connection. The highest-ranking group is used as an ending and all others, in alphabetical order, as prefixes. The different classes of compounds are listed in the following table in order of decreasing priority.
Sometimes there are different names depending on whether the specified carbon atom represents part of the parent system (see keyword nomenclature ) (representation: RC XYZ ) or not (representation: R -CXYZ ). As usual in chemistry, the letter R stands for an organic residue . The placeholder X stands for halides .
| Semi-structural formula | Structural formula | Substance class | Prefix | Ending (suffix) | Examples |
|---|---|---|---|---|---|
| R • or R : | radical | Ylo- | -yl -ylidene |
z. B. Cl • or triplet carbene | |
| Cations | -ium -onium -ylium |
z. B. Cationic surfactants , methyltriphenylphosphonium |
|||
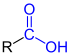
| R- COOH R-C OOH |
Carboxylic acids | Carboxy - |
-carboxylic acid |
z. B. formic acid , acetic acid , butyric acid , amino acids |
|
| R- COOOH R-C OOOH |
Peroxycarboxylic acids | Hydroperoxycarbonyl - |
-peroxycarboxylic acid -peroxy .... acid |
z. B. peroxyacetic acid | |
| R- CS-OH R-C S-OH R- CO-SH R-C O-SH R- CS-SH R-C S-SH |
Thiocarboxylic acids | Hydroxy (thiocarbonyl) - thiocarboxy- - sulfanylcarbonyl- thiocarboxy- - (dithiocarboxy) - - |
-carbothio- O acid thio O acid -carbothio- S acid thio S acid -carbodithiosäure -dithiosäure |
||
| R- SO 2 -OH | Sulfonic acids | Sulfo- | sulfonic acid | z. B. benzenesulfonic acid | |
| R- SO-OH | Sulfinic acids | Sulfino | sulfinic acid | ||
| R 1 - CO-S -R 2 | Thiolester | R 1 oyl -thio- | S -R 2 yl -R 1 thioate | ||
| R- S-OH | Sulfenic acids | Sulfeno | sulfenic acid | ||
| R 1 - SO - R 2 | Sulfoxides | sulfinyl | sulfoxide | z. B. dimethyl sulfoxide | |
| R- COO - M + R-C OO - M + |
Carboxylic acid salts | Metallcarboxylato- - |
Metal -...- carboxylate Metal -...- oat |
z. B. acetate | |
| R - SO 2 - O - M + | Sulfonic acid salts | Metal sulfonato | Metal -...- sulfonate | ||
| R- SO-O - M + | Sulfinic acid salts | Metal sulfinato | Metal -...- sulfinate | ||
| R- S-O - M + | Sulfenic acid salts | Metal sulfenato | Metal -...- sulfenate | ||
| R 1 - CO-O-CO -R 2 | Carboxylic acid anhydrides | - | ...- acid ...- acid anhydride with the same acids: ...- acid anhydride |
z. B. acetic anhydride | |
| R 1 - CO-O -R 2 | Carboxylic acid ester | (R yl) oxycarbonyl |
(R yl -) ...- carboxylate (R yl -) ...- oat - |
z. B. ethyl acetate | |
| R 1 - SO 2 - O - R 2 | Sulfonic acid ester | (R yl-) oxysulfonyl- | (R yl -) ... sulfonate | ||
| R- O-NO 2 | Nitric acid ester | z. B. nitroglycerin , nitrocellulose | |||
| R- CO-X R-C O-X |
Carboxylic acid halides | Halocarbonyl - |
carbonyl halide oyl halide |
z. B. benzoyl chloride | |
| R- SO 2 -X | Sulfonic acid halides | Halosulfonyl | sulfonyl halide | ||
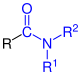
| R- CO-NH 2 R-C O-NH 2 |
Carboxamides | Carbamoyl - |
-carboxamid -amide |
||
| R- SO 2 -NH 2 | Sulfonic acid amides | Sulfamoyl | -sulfonamide | ||
| R- CO-NH-NH 2 R-C O-NH-NH 2 |
Carboxylic acid hydrazides | Hydrazinocarbonyl | -carbohydrazide -ohydrazide |
||
| R- C≡N R- C≡N |
Nitriles | Cyan - |
carbonitrile nitrile |
z. B. acetonitrile | |
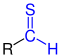
| R- CO-H R-C O-H |
Aldehydes | Formyl oxo- |
-carbaldehyd -al |
z. B. formaldehyde , acetaldehyde | |
| R- CS-H R-C S-H |
Thioaldehydes | Thioformyl- thioxo |
-carbothialdehyde -thial |
||
| R 1 - CO - R 2 | Ketones | Oxo- | -on | z. B. acetone | |
| R 1 - CS -R 2 | Thioketones | Thioxo- | -thione -thioketone |
||
| R 1 R 2 - C = N-OH | Oximes | Hydroxyimino | -aloxime - (carb) aldehyde oxime -onoxime |
||
| R 1 R 2 - C = N - NH 2 | Hydrazones | Hydrazono | -alhydrazone - (carb) aldehydhydrazone -onehydrazone |
||
| R - OH | Alcohols and phenols | Hydroxy | -oil |
Alcohols e.g. B. methanol , ethanol , isopropanol , phenol |
|
| R - SH | Thiols | Sulfanyl- old: mercapto- |
thiol mercaptan |
Thioalcohols , thiophenols e.g. B. methyl mercaptan |
|
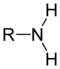
| R - NH 2 | Amines | Amino | -amin | z. B. aniline , amino acids | |
| R 1 R 2 - C = N -R 3 | Imine | Imino | -imin | ||
| R- NH-NH 2 | Hydrazines | Hydrazino | -hydrazine | ||
| R 1 - O - R 2 | Ether | (R 2 yl) -oxy- | (R 1 yl) - (R 2 yl) ether | z. B. diethyl ether , dioxane | |
| R 1 - S -R 2 | Thioether | (R 2 yl) -thio- | (R 1 yl) - (R 2 yl) sulfide | ||
| R- (O) C-NR-C (O) -R | Imide |
Functional groups without priority
The following functional groups are generally only used as prefixes:
| Semi-structural formula | Structural formula | Substance class | prefix | Examples |
|---|---|---|---|---|
| R- X | Halogenated hydrocarbons | Halogen- |
Chlorofluorocarbons e.g. B. Frigen , chloroform |
|
| R- NO 2 | Nitro compounds | Nitro |
Nitrites e.g. B. Trinitrotoluene (TNT) |
|
| R- NO | Nitroso compounds | Nitrosyl | ||
| R 1 - NN - R 2 | Azo compounds | Azo |
Azo dyes e.g. B. Yellow Orange S |
|
| R 1 R 2 - CNN | Diazo compounds | Diazo | z. B. diazomethane | |
| R– NN + Z - | Diazonium salts | Diazonium | ||
| R - NC | Isocyanides | Isocyanate | ||
| R- OCN | Cyanates | Cyanato | ||
| R- NCO | Isocyanates | Isocyanato | ||
| R- SCN | Thiocyanates | Thiocyanato | ||
| R- NCS | Isothiocyanates | Isothiocyanato | ||
| R- OOH | Hydroperoxides | Hydroperoxy | ||
| R 1 - OO - R 2 | Peroxides | (R-) dioxy- |
Special case of multiple ties
The multiple bonds between two carbon atoms are also counted among the functional groups :
| Semi-structural formula | Surname | prefix | Ending | Examples |
|---|---|---|---|---|
| R- C = C- R | Double bond | "-enyl" | "-En" | z. B. Ethene |
| R- C≡C -R | Triple bond | "-inyl" | "-in" | z. B. ethine |
From the nomenclature point of view , multiple bonds are generally regarded as part of the parent system.
Alternative nomenclature systems
In addition to this nomenclature system, there are other, less common or outdated nomenclature systems. One of them is e.g. B. the radical functional nomenclature , according to the z. B. chloromethane can be designated as methyl chloride or ethanol as ethyl alcohol .
Individual evidence
- ↑ Entry on functional group . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.F02555 .
- ^ Rainer Beckert et al .: Organikum . 22nd edition. Wiley-VCH, Weinheim 2004, ISBN 3-527-31148-3 , p. 137.
literature
- Günter Baars, Roland Franik, Walter Jansen, Hans Pickel, Georg Schwedt, Herbert Sommerfeld: Handbook of experimental chemistry: Secondary level II. Volume 10: Functional groups, fats, dyes. (Editors: Walter Jansen, Rudolf G Weissenhorn, Wolfgang Glöckner), Aulis, Hallbergmoos 2008, ISBN 978-3-7614-2388-2 .
- Dieter Hellwinkel: The systematic nomenclature of organic chemistry: Instructions for use . 4th edition. Springer, Berlin 1998, ISBN 3-540-63221-2 .