Thioaldehydes
| Thioaldehydes |
|---|
|
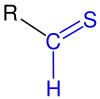
| The thioarbonyl group is marked in blue . R = H, organic radical ( alkyl , aryl or the like). |
Thioaldehydes (Thiale) are organic , chemical compounds . They represent the sulfur analogues of aldehydes and belong to the thiocarbonyl compounds .
Their functional group consists of a thiocarbonyl carbon atom to which a sulfur atom with a double bond and a hydrogen atom with a single bond is bonded. Thioaldehydes are much more reactive than aldehydes . Their monomers often form trimers, oligo- and polymeric products. However, they can be stabilized thermodynamically or kinetically . Thioaldehydes are of little technical importance, but they do occur naturally. For example, thioacrolein is created by the breakdown of allicin in garlic .
properties
Thioaldehydes easily trimerize to trithianes . The trimer of thioacetaldehyde can e.g. B. occur in the α-form or in the β-form:
| Isomerism of trithianes (here 2,4,6-trimethyl-1,3,5-trithian) | |
|---|---|
 α-form |
 β-form |
The rearrangement of the α-form into the β-form - and vice versa - can be promoted by catalysts such as iodine , sulfuric acid , hydrochloric acid or acid chlorides . In the case of aromatic thioaldehydes, the tendency to trimerization is lower.
The stabilization of thioaldehydes can be done either sterically by spatially large residues ( kinetic stabilization ), such as. B. in the case of 2,4,6-tri- tert -butylthiobenzaldehyde. It does not trimerize or polymerize because of the sterically demanding residues. Another possibility is thermodynamic stabilization , with suitable substituents such as amines , alcoholates , thiolates or vinyl groups ( vinylogy principle ) increasing the mesomeric stability and thereby reducing the instability of the compound. Thermodynamically stabilized thioaldehydes are considered to be particularly stable and isolatable. Stable aliphatic thioaldehydes are usually pink and can be crystalline or as oils. Stable aromatic thioaldehydes can appear purple, dark blue, or green and are mostly crystalline.
synthesis
The synthesis of thioaldehydes is difficult because of the hydrogen atom attached to the thiocarbonyl carbon atom . Nevertheless, some synthetic routes are formulated in the literature. A common method is the pyrolysis of trithianes. If this z. B. 1,3,5-trithiane is used, thioformaldehyde is formed:
The thioaldehydes formed are recombined to form new trithianes. Accordingly, differently substituted trithians produce differently substituted products. An alternative is the reaction of acetaldehyde (or other aldehydes ) with sulfur and butyllithium substituted by trimethylsilyl , which leads to thioacetaldehyde. A similar reaction can be carried out with cobalt (II) chloride .
The resulting thioaldehydes are unstable. Their existence is proven by the subsequent Diels-Alder reaction with cyclopentadiene . The conversion of thioaldehydes into Diels-Alder adducts is one of the common processes because these cycloadditions are often a reversible reaction . Thioaldehydes are generally reactive dienophiles with 1,3-dienes in Diels-Alder reactions. This can also be done intramolecularly. The Diels-Alder reaction is also possible with photometrically generated thioaldehydes on polymer surfaces. Thioaldehydes can also be effectively obtained by the pyrolysis of allyl sulfides.
Reactions
Thioaldehydes react with nucleophiles largely in the same way as aldehydes. The reactivity caused by their instability often leads to trimers , oligomers and polymers .
The oxidation of thioaldehydes is carried out with meta-chloroperbenzoic acid and leads to sulfines. By reduction with sodium borohydride or lithium aluminum hydride are thiols received. In addition, other reactions are described in the literature.
See also
Individual evidence
- ↑ a b Entry on thioaldehydes. In: Römpp Online . Georg Thieme Verlag, accessed on June 3, 2020.
- ↑ a b c R. Kracher among others: Lexikon der Chemie. Volume 3: Perf to Zy. Jokers edition. Spektrum Verlag, Heidelberg 2007, ISBN 978-3-8274-1909-5 , p. 338.
- ↑ HW Kroto et al .: The photoelectron and microwave spectra of the unstable species thioacetaldehyde, CH 3 CHS, and thioacetone, (CH 3 ) 2 CS. In: Chemical Physics Letters . Volume 29, No. 2, 1994, pp. 265-269, doi: 10.1016 / 0009-2614 (74) 85029-3 .
- ↑ A. Schönberg, A. Wagner: Methods for the production and conversion of thioaldehydes and thioketones. In: E. Müller (Ed.): Methods of Organic Chemistry . Volume IX: sulfur, selenium, tellurium compounds. Thieme Verlag, Stuttgart 1955, pp. 699-703.
- ↑ a b J. Voss: Thioaldehydes or Thioketones. In: D. Klamann (Ed.): Methods of Organic Chemistry . Volume E11: Organic Sulfur Compounds. Thieme Verlag, Stuttgart 1985, ISBN 3-13-218104-8 , pp. 188-194.
- ↑ a b c d e R. Okazaki: Chemistry of Thioaldehydes. In: P. Page (Ed.): Organosulfur Chemistry. Volume 1, Academic, San Diego 1995, pp. 225-258.
- ^ A b c Siegfried Hauptmann : Organic Chemistry. 2nd, revised edition. VEB Deutscher Verlag für Grundstofftindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 389.
- ^ H. Morita: Formation of thioaldehyde intermediates by thermolysis of sulfoxides bearing some heteroaromatics. In: The Journal of Organic Chemistry . Volume 62, No. 26, 1997, pp. 9018-9023, doi: 10.1021 / jo9700181 .
- ↑ GW Kirby: Thioaldehydes in synthesis. In: Phosphorus, Sulfur, and Silicon and the Related Elements. Volume 74, No. 1-4, 1993, pp. 17-29, doi: 10.1080 / 10426509308038098 .
- ^ WM McGregor, DC Sherrington: Some Recent Synthetic Routes th Thioketones and Thioaldehydes. In: Chemical Society Reviews . Volume 22, No. 3, 1993, pp. 199-204, doi: 10.1039 / CS9932200199 .
- ↑ M. Segi et al: Intramolecular Diels-Alder Reaction of Thioaldehydes. In: Synthetic Communications . Volume 19, No. 13-14, 1989, pp. 2431-2439, doi: 10.1080 / 00397918908052644 .
- ↑ M. Glassner et al.: Polymer surface patterning via Diels-Alder trapping of photo-generated thioaldehydes. In: Chemical Communications . Volume 49, No. 6, 2013, pp. 633-635, doi: 10.1039 / C2CC37651B .
- ↑ T. Pauloehrl et al .: Spatially controlled surface immobilization of nucleophiles via trapping of photo-generated thioaldehydes. In: Chemical Science . Volume 4, No. 9, 2013, pp. 3503-3507, doi: 10.1039 / C3SC50815C .
- ↑ UAE Usov, LV Timokhina, MG Voronkov: The synthesis and properties of thioaldehydes. In: Russian Chemical Reviews . Vol. 59, No. 4, 1990, pp. 378-395, doi: 10.1070 / RC1990v059n04ABEH003531 .
- ↑ G. Opitz: Sulfine and Sulfene - the S-oxides and S, S-dioxides of thioaldehydes and thioketones. In: Angewandte Chemie . Volume 79, No. 4, 1967, pp. 161-177, doi: 10.1002 / anie.19670790402 .
- ↑ K. Okuma: Recent Studies on the Reactions of Thioaldehydes and Thioketones. In: Sulfur reports. Volume 23, No. 2, 1990, pp. 209-241, doi: 10.1080 / 01961770208047971 .
- ↑ G. Li et al .: On the behavior of α, β-unsaturated thioaldehydes and thioketones in the dielectric reaction. In: The Journal of Organic Chemistry . Volume 65, No. 20, 2000, pp. 6601-6612, doi: 10.1021 / jo000740q .




