Thiolates
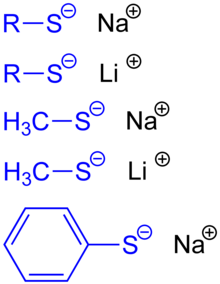
Thiolates (obsolete also Thioalkoholate or mercaptides are called) in the chemical salts of metal cations and thiolate anions , so anions with sulfur , the to organic radicals is bonded. The general formula is (RS) n Me (R = remainder, S = sulfur, Me = metal ion; n corresponds to the valence of this metal ion). Thiolate ions are formed by deprotonation from thiols (thioalcohols, mercaptans).
presentation
Thiolates can be synthesized by reacting elemental alkali metals with thioalcohols (thiols) . Other bases, such as sodium amide , can also be used here. Thiolates can even be produced in an aqueous medium, since the hydroxide ion is sufficiently basic to deprotonate the thiol group and so alkali hydroxides can be reacted with thioalcohols. For example, sodium thiophenolate is formed by the action of sodium hydroxide on thiophenol :
properties
In their pure form, thiolates are strongly basic hygroscopic solids, so they attract moisture (water) from the air and gradually dissolve . They are more resistant to water than the alcoholates . Lead (II) and mercury (II) thiolates are sparingly soluble and, like other heavy metal captides, are covalent compounds.
Usage and reactions
Thiolates are used in the synthesis of thioethers ( sulfides , RSR). In this process (analogous to the Williamson ether synthesis ) a halogenoalkane is reacted with a thiolate in a nucleophilic substitution .
Analytical evidence
The reaction of thiolates with 1-chloro-2,4-dinitrobenzene gives the corresponding 2,4-dinitrophenyl sulfides, which often have a characteristic melting point :
See also
- Alcoholates (oxygen analogues of thioalcoholates)
Individual evidence
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag, Leipzig 1965, p. 860.
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, p. 475, ISBN 3-342-00280-8 .

