Sodium amide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
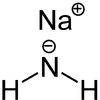
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium amide | |||||||||||||||
| Molecular formula | NaNH 2 | |||||||||||||||
| Brief description |
off-white solid with an ammonia-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 39.01 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.39 g cm −3 |
|||||||||||||||
| Melting point |
210 ° C |
|||||||||||||||
| boiling point |
400 ° C |
|||||||||||||||
| solubility |
Decomposes in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium amide is a colorless, coarsely crystalline or powdery compound with the composition NaNH 2 .
presentation
Sodium amide can be prepared on a laboratory scale from metallic sodium and liquid ammonia in the presence of iron (III) ions with the release of hydrogen .
Without iron catalyst is not the amide formed (unlike the analog synthesis of sodium from sodium and water), instead it comes to the solvation of the 3s valence electron of sodium in ammonia. The free electrons lead to a deep blue coloration of the solution , which can be understood as an ammoniacal solution of the hypothetical “ salt ” Na + e - . If the solution is evaporated, Na + is reduced again to the element , the yield of NaNH 2 is only less than 0.5%.
properties
Sodium amide is a colorless, hygroscopic solid. It is a very strong base that is suitable for deprotonating only weakly acidic compounds.
Responsiveness
Sodium amide reacts violently with water to form sodium hydroxide and ammonia. The severity of the reaction is comparable to that of sodium and water.
Sodium amide is slowly oxidized by atmospheric oxygen , with the formation of sometimes explosive reaction products such as sodium hyponitrite, sodium trioxodinitrate, sodium tetraoxodinitrate and higher peroxonitrates. The oxidation products are extremely sensitive to friction and impact . Even simple grinding, crushing and decanting work can lead to violent explosions. The formation of unstable oxidation products can be recognized by the discoloration of the substance. It is therefore advisable to keep the connection in a protective liquid ( petroleum ) or, better yet, to use it immediately. Old, oxidized preparations can no longer be used and must be disposed of with reasonable precautions.
use
Sodium amide is used in organic chemistry as a strong base for deprotonating less acidic compounds. For example, the highly strained arynes can be obtained by reacting bromobenzene derivatives with sodium amide in an E1 cb reaction . Seldom, sodium amide is also used to deprotonate alcohols or terminal alkynes .
Sodium acetylide obtained from acetylene by deprotonation can be used for alkynylation. Since sodium amide is not sterically demanding, it is also used for the deprotonation of sterically poorly accessible protons .
It can also be used to make sodium azide .
As a nucleophile , it acts in the Chichibabin reaction , in which it nucleophilically on a pyridine to form ring amino engages pyridines.
literature
- K. Peter C. Vollhardt and Neil E. Schore: Organic chemistry 3rd edition. Wiley-VCH, 2000, ISBN 3-527-29819-3 .
Individual evidence
- ↑ a b c d e f g Entry on sodium amide in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b Jander, Blasius, Strähle: Introduction to the inorganic chemical internship . 14th edition. Hirzel, Stuttgart 1995, ISBN 978-3-7776-0672-9 , pp. 204-205.
- ↑ G. Brauer (Ed.): Handbook of Preparative Inorganic Chemistry 2nd ed., Vol. 1, Academic Press 1963, pp. 465-8.
- ↑ a b c d e f Roth / Weller: Hazardous chemical reactions , supplementary delivery 08/2007, ecomed SECURITY, publishing group Hüthig Jehle Rehm, Landsberg / Lech
- ↑ Entry on sodium amide. In: Römpp Online . Georg Thieme Verlag, accessed on February 3, 2018.






