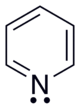
Pyridine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Pyridine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 5 N | |||||||||||||||
| Brief description |
colorless, hygroscopic liquid with an unpleasant, characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 79.10 g · mol -1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.98 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−42 ° C |
|||||||||||||||
| boiling point |
115 ° C |
|||||||||||||||
| Vapor pressure |
20.5 hPa (20 ° C) |
|||||||||||||||
| pK s value |
5.23 (conjugate acid at 25 ° C) |
|||||||||||||||
| solubility |
miscible with water, ethanol , acetone , chloroform , diethyl ether and benzene |
|||||||||||||||
| Dipole moment |
2.2 D |
|||||||||||||||
| Refractive index |
1.5095 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
100.2 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Pyridine is a colorless and highly flammable chemical compound with the empirical formula C 5 H 5 N. It belongs to the heterocyclic parent systems and forms the simplest azine , which consists of a six-membered ring with five carbon atoms and one nitrogen atom . The name azine is derived from the systematic Hantzsch-Widman nomenclature , according to which pyridine is called azine . In analogy to benzene , the name azabenzene is occasionally used. In 1849, pyridine was first described by the Scottish chemist and physician Thomas Anderson , who studied the ingredients of bone oil . Two years later, Anderson isolated pyridine for the first time in pure form by fractional distillation of the oil.
In the chemical industry , pyridine is both an important synthesis component for the manufacture of drugs or herbicides and a common solvent for chemical reactions . Tens of thousands of tons of the compound are produced worldwide every year and largely reused in the chemical industry. Historically, pyridine was obtained from tar or as a by-product of coal gasification ; However, due to the increased demand, these methods have given way to more economical synthetic processes over the years.
Pyridine meets the Hückel criteria for aromaticity and has typical heteroaromatic properties. Its reactivity to electrophilic substitutions is markedly reduced compared to the homoaromatic analogue benzene, whereas nucleophilic substitutions occur more frequently.
history
Pyridine was undoubtedly obtained in an impure form by heating animal material as early as alchemical times. The earliest written mention in 1851 is to be attributed to the Scottish natural scientist Thomas Anderson (1819–1874). He investigated the ingredients of bone oil , which is obtained by vigorously heating dry bones. Among other things, he received a colorless, foul-smelling liquid that he was able to isolate in pure form for the first time two years later.
“The first of these bases, which I want to call pyridine, is contained in the portion that passes over at about 115 ° C. This portion has a very similar smell to that of picolin , but is even stronger and more pungent. It is completely transparent and colorless and does not change color in contact with the air. It is in every proportion in water and easily soluble in volatile and non-volatile oils. In concentrated acids it dissolves under intense heat development, and forms very easily soluble salts with them. "
The name, which is derived from the Greek πυρος ( pyros ) = fire, was given to pyridine analogously to the already known nitrogen base pyrrole , since the first isolation also took place at high temperatures. The ending -in was chosen in accordance with the already established organic bases aniline and toluidine .
The chemical structure of pyridine could only be finally elucidated decades later. Körner and Dewar independently postulated the hypothesis that there was an analogy between benzene and naphthalene as well as pyridine and quinoline ; in the structures of the former, only one CH unit had to be replaced by a nitrogen atom. This could be proven by reducing pyridine using metallic sodium to piperidine , the structure of which was already known at that time.
In 1877, William Ramsay passed acetylene and hydrogen cyanide gas through a red - hot pipe, producing pyridine. This makes pyridine one of the first synthetically produced heteroaromatic compounds.
In the decades that followed, the demand for pyridine grew, which is why synthetic methods for its extraction were developed. A breakthrough came with the Russian chemist Alexei Evgenjewitsch Tschitschibabin , who in 1924 developed an economical synthesis route from inexpensive synthesis building blocks that is still used today for industrial production.
Occurrence
Few natural occurrences of free pyridine are known. However, it could be detected in the volatile components of the marshmallow as well as the leaves and roots of the black belladonna ( Atropa belladonna ). Its derivatives , on the other hand, are often part of biomolecules such as the pyridine nucleotides named after him and natural oils and gases.
Pyridine is produced by roasting and preservation processes in food and can be detected in small amounts in its volatile components. These include fried chicken , sukiyaki , fried ham , Beaufort cheese , coffee flavoring , black tea, and sunflower honey. Both the smoke from tobacco and marijuana contain pyridine.
nomenclature
The systematic name of pyridine according to the Hantzsch-Widman system , which is recommended by the IUPAC , is azine . In the field of heterocycle nomenclature, however, historically common trivial names are often used, which is why the systematic designation is neither common in linguistic usage nor in specialist literature. Contrary to the systematic, IUPAC explicitly recommends keeping the name pyridine . The numbering of the ring atoms begins at the nitrogen atom, which has the highest priority, and continues from 2 to 6 via the carbon ring members. An assignment of the positions by letters of the Greek alphabet (α – γ) and the substitution pattern nomenclature that is common in homoaromatic systems ( ortho , meta , para ) can also be found in some cases.
The systematic name of the pyridine residue is pyridinyl , with the position of the link as a number. However, in this case too, pyridine is an exception to the system, as the historically common pyridyl is recommended as a name. The cationic pyridine residue that results from the addition of an electrophile to the nitrogen atom is called pyridinium .
2,6-pyridinedicarboxylic acid ( dipicolinic acid )
Basic structure of pyridinium compounds
Extraction and presentation
Historically, pyridine was obtained from tar or from coal gasification. However, coal tar only contains about 0.1% pyridine, which can be expelled from the raw substance as a mixture with other substances. However, multi-stage purification processes are necessary to separate the mixture, which is why such a process is no longer economical in view of the low yield. Today almost all global demand is met by synthetic pyridine.
Chichibabin pyridine synthesis
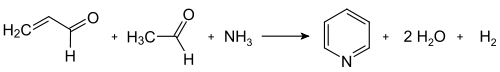
Modern industrial syntheses use the route published by Tschitschibabin for the first time in 1924, which is a multicomponent reaction between ketones or aldehydes with ammonia . The synthesis of the unsubstituted pyridine requires formaldehyde and acetaldehyde - inexpensive synthetic building blocks that are available on a multi-ton scale. In an aldol condensation , acrolein is initially formed from a portion of the aldehydes , which is condensed with acetaldehyde and ammonia to 1,4-dihydropyridine and then oxidized to pyridine on the solid-phase catalyst. Technically, this is carried out as a gas phase reaction at 400-450 ° C. The composition of the product mixture, consisting of pyridine, monomethylated pyridines ( picolines ) and lutidines , depends on the catalyst used and can be adapted to the needs of the manufacturer. Transition metal salts such as cadmium fluoride and manganese (II) fluoride on silicate carriers serve as catalyst materials, but cobalt and thallium compounds can also be used. The pyridine obtained can be separated from the by-products in a multi-stage process and these can either be further processed or converted into pyridine by demethylation.
Dealkylation of alkyl pyridines
Pyridine can be produced by dealkylation of alkylated pyridines, which occur as by-products in common industrial syntheses. Dealkylation takes place either oxidatively with air on the vanadium oxide catalyst, by steam dealkylation on the nickel catalyst or by hydrodealkylation on the silver or platinum catalyst. Yields of pyridine of up to 93% on the nickel catalyst are possible here.
Hantzsch pyridine synthesis
A first important synthetic route to pyridine derivatives was described in 1881 by Arthur Hantzsch . A β-keto ester (often acetoacetic ester ), an aldehyde (often formaldehyde ) and ammonia or ammonium salts in a ratio of 2: 1: 1 are used ( Hantzsch dihydropyridine synthesis ). A doubly hydrogenated pyridine is first obtained, which can be converted oxidatively to the corresponding pyridine derivative in a subsequent step . Knoevenagel showed that asymmetrically substituted pyridine derivatives can also be obtained in this way.

Bönnemann cyclization
The trimerization of one part of the nitrile component and two parts of acetylene is called the Bönnemann cyclization according to Helmut Bönnemann . This is a modification of the Reppe synthesis , which can be carried out both thermally and photochemically . While high pressures and temperatures are required for the thermal reaction, the photoinduced cycloaddition can even be carried out in water under normal conditions with the catalytic use of CoCp 2 (cod) (Cp = cyclopentadienyl, cod = 1,5-cyclooctadiene ). A number of pyridine derivatives are accessible in this way. If acetonitrile is used as the nitrile component, 2-methylpyridine is obtained, which can be dealkylated to pyridine.
Biosynthesis of the pyridine ring
Several pyridine derivatives sometimes play a prominent role in biological systems. The exact biosynthetic structure of the pyridine ring depends on the biological system and the exact structure of the pyridine derivative. While the biosynthetic access of many pyridine derivatives has not yet been fully clarified, the synthetic route of the pyridine derivative nicotinic acid (vitamin B 3 ) in some bacteria , fungi and mammals is considered safe. Mammals often synthesize nicotinic acid by oxidative degradation of the amino acid tryptophan , whereby the aniline derivative kynurenine is formed as an intermediate product . In the bacteria Mycobacterium tuberculosis and Escherichia coli , on the other hand, glyceraldehyde-3-phosphate and aspartic acid are required for biosynthesis .
properties
Physical Properties
| Critical sizes | ||
|---|---|---|
| pressure | temperature | volume |
| 6.70 M Pa | 620 K | 229 cm 3 mol −1 |
| Parameters for the Antoine equation (340-426 ° C) | ||
| A. | B. | C. |
| 4.16272 | 1371.358 | −58.496 |
|
Temperature dependence of the vapor pressure (according to Δ V H 0 = A exp (−β T r ) (1 − T r ) β ) between 298 and 388 ° C |
||
| A. | β | T c |
| 55.43 kJ mol −1 | 0.2536 | 620 K |
Pyridine is colorless and liquid under standard conditions, boiling at 115.23 ° C and freezing at −41.70 ° C. It is a highly light-refractive liquid that has a refractive index of 1.5095 at 20 ° C and a wavelength of 589 nm . Under standard conditions, pyridine has a density comparable to that of water of 0.9819 g · cm −3 . Pyridine has an electrical dipole moment of 2.2 D , is diamagnetic and has a molar diamagnetic susceptibility of −48.7 · 10 −6 cm 3 · mol −1 . In the liquid phase the standard enthalpy of formation is 100.2 kJ mol −1 , whereas in the gas phase it is 140.4 kJ mol −1 . At 25 ° C, pyridine has a viscosity of 0.879 mPa · s −1 and a thermal conductivity of 0.166 W · (m · K) −1 . A vapor pressure of 20.5 h Pa results under standard conditions . The enthalpy of vaporization at the boiling point under normal pressure is 35.09 kJ mol −1 . A melting enthalpy of 8.28 kJ · mol −1 is achieved at the melting point .
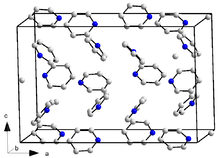
Pyridine crystallizes in the orthorhombic crystal system in the space group Pna 2 1 (space group no. 33) with the lattice parameters a = 1752 pm , b = 897 pm and c = 1135 pm and 16 formula units per unit cell . A crystalline trihydrate (pyridine · 3 H 2 O) is also known. This also crystallizes in the orthorhombic crystal system, but in the space group Pbca (No. 61) with the lattice parameters a = 1244 pm , b = 1783 pm and c = 679 pm and eight formula units per unit cell.
Chemical properties
Pyridine is miscible with water, ethanol , diethyl ether , acetone , benzene and chloroform . It has a weakly basic reaction and forms a crystalline hydrochloride with hydrochloric acid (hydrochloric acid) , which only melts at 145–147 ° C.
Pyridine belongs to the class of heteroaromatics and has typical properties of this class of substances. However, due to the influence of the electronegative nitrogen , the pyridine ring is relatively electron deficient, which means that the electrophilic substitution reaction typical of aromatic systems is inhibited. Compared to its carbon analogue, benzene, pyridine shows a significantly lower reactivity with regard to electrophilic aromatic substitutions. In contrast to carbon aromatics, however, pyridine has a comparatively higher reactivity with regard to nucleophilic substitutions and the metalation of the ring by strongly basic organometallic compounds . The reactivity of pyridine exhibits characteristics of three chemical groups. With electrophiles, electrophilic substitutions take place, in which the aromatic properties of pyridine are expressed. Pyridine reacts with nucleophiles in the 2- and 4-position and thus has similarities with the reactivity of imines or carbonyl compounds . The reaction with many Lewis acids leads to addition to the nitrogen atom, whereby pyridine shows similarities to the reactivity of tertiary amines. The ability to form N -oxides through oxidation is also a characteristic of tertiary amines.
Pyridine forms complexes with numerous transition metal ions . Here , pyridine coordinates with the lone pair of electrons on the nitrogen atom to the metal center. η 6 coordination, as occurs with benzene, is only possible through steric blocking of the nitrogen atom.
Molecular Properties
Pyridine has a fully conjugated system of six π electrons that are delocalized over the entire ring system . Furthermore, pyridine is built in a planar manner and thus follows the Hückel criteria for aromatic systems. In contrast to benzene, however , the electron density is not evenly distributed, which is due to the negative inductive effect of the nitrogen atom. For this reason, pyridine has a dipole moment and is less resonance stabilized than benzene (benzene: 150 kJ mol −1 , pyridine: 117 kJ mol −1 ). The higher electron density is also expressed in the shortened bond length of the nitrogen-carbon bond (benzene: 139 pm , pyridine, CN: 137 pm), while the carbon-carbon bonds have the same bond length as in the benzene molecule (139 pm). The bond lengths illustrate the aromatic character of pyridine. As usual for aromatic systems, they lie between the values that are typically expected for single-bonded and double-bonded atoms.

In the pyridine molecule, all ring atoms are sp 2 - hybridized . The nitrogen atom provides the electron of its p orbital for the formation of the aromatic system, its free sp 2 electron pair lies in the molecular plane and points outwards from the ring. This pair of electrons does not contribute to the aromatic system, but is of great importance for the chemical properties of pyridine. Due to the periplanar arrangement of the lone pair of electrons, the aromatic system is not broken by bond formation at this position, which favors an electrophilic attack at this position. The separation of the lone pair of electrons from aromatic systems also means that the nitrogen atom cannot develop a positive mesomeric effect . The reactivity of pyridine is thus largely determined by the negative inductive effect of the nitrogen atom.
Pyridine is resonance-stabilized via five mesomeric boundary structures and for this reason is more stable than the hypothetical 1-aza-1,3,5-cyclohexatriene with localized double bonds . Similar to benzene, there are two boundary structures that do not have a zwitterionic character. In addition, however, three other zwitterionic boundary structures can be formulated which assign a negative charge to the nitrogen atom , whereby the positive charge occurs at the 4-position or one of the two 2-positions of the ring. The position of the charge on the nitrogen atom is consistent with its higher electronegativity compared to carbon .
Reactions
Many of the reactions characteristic of the homologous benzene do not take place on pyridine or only take place under more complex conditions or with poor yield. This is essentially due to the reduced electron density in the aromatic system, which deactivates pyridine and its derivatives for electrophilic substitutions , and the preferred addition of electrophiles to the electron-rich nitrogen atom. The electrophilic addition on the nitrogen atom leads to a further deactivation of the aromatic, which makes subsequent electrophilic substitutions even more difficult. On the other hand, radical and nucleophilic substitutions occur more frequently than with benzene and are often even the preferred reaction route.
Electrophilic substitutions
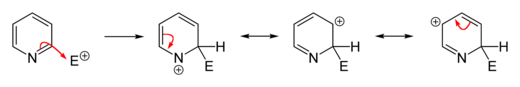
In many cases, electrophilic substitutions on pyridine do not take place or only take place incompletely, but the heteroaromatic can be activated by electron-donating functionalization. Common alkylations and acylations (for example by Friedel-Crafts alkylation or acylation ) usually fail because they only lead to addition at the nitrogen atom. Substitutions usually take place at the 3-position because, on the one hand, it is the most electron-rich carbon atom of the molecule, which facilitates electrophilic addition, and on the other hand, the resulting σ-complex has no boundary structure that assigns a positive charge to the nitrogen atom . This is the case in the case of an addition in the 2- or 4-position and thus causes an energetically less favorable σ-complex.
However, if substituents are to be introduced in the 2- or 4-position, there are established ways of carrying out the reaction accordingly. A variant that is often used is to carry out the electrophilic substitution on the activated N -oxide of pyridine and subsequent deoxygenation of the nitrogen atom. In this variant, products which are substituted in the 2- and 4-position are generally obtained, since the oxygen atom of the N -oxide provides the aromatic system with electron density and thus favors the substitution in these positions compared to a substitution in the 3-position. A number of common reducing agents can be used for deoxygenation . There are generally trivalent phosphorus compounds or divalent sulfur compounds that are easily oxidized. Triphenylphosphine , which is oxidized to triphenylphosphine oxide in the reaction , is often used as a cheap reagent . Selected widespread electrophilic substitutions on pyridine are exemplified below.
The direct nitration of pyridine proceeds only with very low yields, even under drastic conditions. However, 3-nitropyridine can be prepared in another way by reacting pyridine with nitrous oxide and sodium hydrogen sulfite . Pyridine derivatives, which sterically and / or electronically shield the nitrogen atom, can be nitrated directly by nitronium tetrafluoroborate . In this way, the synthesis of 3-nitropyridine from 2,6-dibromopyridine and subsequent dehalogenation succeeds . The sulfonation of pyridine also proceeds with similar success, and takes place without significant conversion even under severe conditions. However, pyridine-3-sulfonic acid is produced by boiling in an excess of oleum at 320 ° C. with an acceptable yield. The reason for this behavior is the preferred addition of the electrophile sulfur trioxide to the pyridine nitrogen, whereby the heteroaromatic is additionally deactivated for the electrophilic attack required to introduce the sulfonic acid group . However, the sulfonation with oleum proceeds smoothly in the presence of catalytic amounts of mercury (II) sulfate . The underlying mechanism has not yet been clarified.
In contrast to nitration and sulfonation, the bromination and chlorination of pyridine can be carried out directly. The conversion of pyridine with molecular bromine in oleum at 130 ° C to 3-bromopyridine proceeds with very good yield, whereas the chlorination with molecular chlorine in the presence of aluminum chloride at 100 ° C to 3-chloropyridine only with moderate yield. In the presence of catalytic amounts of palladium (II) chloride , 2-bromopyridine and 2-chloropyridine are also preparatively accessible by reaction with the molecular halogens .
Nucleophilic Substitutions
In contrast to benzene, a number of efficient nucleophilic substitutions on pyridine are known. The reason for this is the comparatively lower electron density of the heteroaromatic, which favors attacks by nucleophiles . Both ipso -substitutions on leaving groups -bearing ring atoms and reactions with elimination of hydride ions as well as elimination-addition reactions via heteroarine intermediates occur. They mostly supply the products substituted in the 2- or 4-position.
In many cases , ipso substitutions take place smoothly on pyridine derivatives that have good leaving groups . Mostly bromine-, chlorine- or fluorine-substituted substrates are used for this, but the sulfonic acid group can also serve as a leaving group. For substitution with organolithium compounds , fluorine is the best leaving group. In addition, alcoholates , thiolates, but also amines , and at elevated pressure , ammonia, can also be used as nucleophiles .
The hydride ion is generally a very poor leaving group. In heterocycle chemistry, however, few reactions are known in which the hydride ion acts as a leaving group. These include the Tschitschibabin reaction , by means of which pyridine derivatives aminated in the 2-position can be produced. Sodium amide is used as the nucleophile for this purpose , which adds to pyridine in the 2-position and, after aqueous work -up, releases 2-aminopyridine . The hydride ion is split off from the pyridine ring in the course of the reaction and forms molecular hydrogen with a proton of another amino group .
When using lithium organyls as nucleophiles, these can add directly to pyridine due to the directing effect of the nitrogen atom, preferably in the 2-position. Depending on the nucleophile and substrate used, lithium hydride is subsequently split off directly . However, if the intermediate N lithium salt is persistent, oxidative conditions must be created for rearomatization with the release of the substituted pyridine.
Analogously to benzene, the formation of hetero arynes is possible as an intermediate. For this purpose, pyridine derivatives with good leaving groups are eliminated with strong bases such as sodium amide and potassium tert -butanolate to form the heteroarin . The subsequent addition of a nucleophile to the triple bond usually proceeds with low selectivity and a mixture of the two possible addition products is obtained.
Radical reactions
Various radical reactions take place on pyridine. Here, the dimerizations of pyridine to bipyridines are of preparative interest . The radical dimerization of pyridine with elemental sodium or Raney nickel selectively yields 4,4'-bipyridine and 2,2'-bipyridine , which are important raw materials in the chemical industry. As named reactions are free radical reactions under acidic conditions to heteroaromatics as Minisci reaction known. On pyridine, these lead with high selectivity to the products substituted in the 2- or 4-position. Thus, 2- tert- butylpyridine can be obtained from pyridine by reacting with pivalic acid , silver nitrate and ammonium peroxodisulfate in sulfuric acid solution with a yield of 97% by a Minisci reaction.
Reactions at the nitrogen atom
Lewis acids easily add to the nitrogen atom of pyridine, forming pyridinium salts. With hydrohalic acids , the corresponding hydrochlorides or hydrobromides , which are of greater importance, are obtained analogously . The reaction with alkyl halides results in the alkylation of the nitrogen atom. This creates a positive charge in the ring, which strongly influences the reactivity of pyridine and facilitates both oxidation and reduction reactions. The Zincke reaction can be used for the selective introduction of residues of pyridinium compounds, whereby the underlying primary amines are required.
The reaction with secondary amines, however, leads to ring opening, whereby Zincke aldehydes are obtained.
Hydrogenation and reduction
The saturated piperidine is obtained by complete hydrogenation using hydrogen in the presence of Raney nickel . A heat of reaction of −193.8 kJ mol −1 is released. This is somewhat lower than the heat of hydrogenation of benzene at −205.3 kJ mol −1 .
Partially hydrogenated derivatives can be obtained under milder conditions. Reduction by means of lithium aluminum hydride gives a mixture of 1,4-dihydropyridine , 1,2-dihydropyridine and 2,5-dihydropyridine . Pure 1,4-dihydropyridine is formed from pyridine in the presence of organic magnesium and zinc complexes. (Δ3,4) -Tetrahydropyridine can be obtained by electrochemical reduction of pyridine.
use
Today pyridine is an important raw material in the chemical industry , which is produced annually on the kiloton scale (26,000 t / a, as of 1989). There are 25 known production sites for pyridine worldwide, eleven of which are on European soil (as of 1999). The major producers of pyridine include or included Degussa , Rütgerswerke , ICI and Koei Chemical . In recent years, however, the capacity for pyridine production has increased significantly, so that plants with a capacity of 50,000 t / a have been built in China alone . According to its own information, the American-Chinese joint venture Vertellus is currently the world market leader for pyridine.
Pyridine has a wide range of applications in the preparative chemical industry . It is used as a polar , basic, less reactive solvent that is used both as a catalyst , activating agent and as a base for binding acids that are formed. It is particularly suitable for dehalogenation, where it acts as a base for the elimination reaction and binds the resulting hydrohalic acid to form a pyridinium salt. In esterifications and acylations, pyridine can be used to activate the carboxylic acid halides or anhydrides used. However, the pyridine derivatives DMAP and PPY are more active in these reactions . Pyridine can also be used as a base in condensation reactions .
The chromate salt pyridinium chlorochromate (PCC) was developed in 1975 by Elias Corey and William Suggs and serves as a strong oxidizing agent, which is mostly used for the oxidation of alcohols . It is obtained from the reaction of pyridine with hydrochloric acid and chromium (VI) oxide . However, since it is carcinogenic, it should be replaced by less toxic oxidizing agents if possible. The Cornforth - (pyridinium dichromate, PDC) and the Collins reagent are similar chromium -based pyridine compounds, which have the same hazard potential and are also used for oxidation.
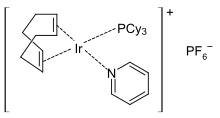
In metal complexes, pyridine is a labile ligand and can easily be exchanged for stronger complexing Lewis bases, which is exploited in catalysis. Pyridine complexes with transition metal ions are used as polymerization or hydrogenation catalysts , for example the Crabtree catalyst . The catalyst species initially carries a pyridine ligand, which is easily exchanged for the substrate. After the end of the catalytic cycle , pyridine coordinates again on the catalyst and thus causes the coordinative saturation of the metal ion.
In the chemical and pharmaceutical industries , pyridine is used as a synthetic building block for the production of a large number of drugs , insecticides and herbicides . Pyridine is or has been used in large quantities to produce the herbicides diquat or paraquat , which have a bipyridine structure. The first step in the synthesis of the insecticide chlorpyrifos consists of the chlorination of pyridine; it is also the starting compound for the production of the fungicide pyrithione . The quaternary pyridinium salts cetylpyridinium chloride and laurylpyridinium chloride , which can be produced from pyridine in a Zincke reaction, are used as antiseptics in oral and dental care products.
In addition to pyridines, derivatives of piperidine are also important synthetic building blocks. A common synthesis of piperidine is the reduction of pyridine. In industrial processes, pyridine can be reduced to piperidine over a nickel , cobalt or ruthenium catalyst at elevated temperature.
Among other things, pyridine is also used as a solvent in dye and rubber production and is used in the textile industry to improve the wetting properties of cotton .
For denaturing of ethanol to methylated spirits which are alcohol mixed with substances which cause it to be unfit for human consumption and are difficult to separate by physical processes. Due to its bitter taste and its physical properties, pyridine was often part of this mixture of substances, but is now mostly replaced by other substances. In small doses, however, pyridine is also used as a bitter flavor in foods. In solution , the detection threshold for pyridine is 1–3 m mol · l −1 (79–237 mg · l −1 ).
As a base, pyridine can be used as a component of the Karl Fischer reagent . In modern reagents, however, it is usually replaced by another base due to the unpleasant smell.
Hazard warnings
Pyridine has a flash point of 17 ° C and is therefore highly flammable. The ignition temperature is given as 550 ° C. In a range of 1.7–10.6% by volume, pyridine forms explosive mixtures with air . The thermal decomposition of pyridine begins above 490 ° C, the decomposition products being bipyridines , essentially 2,2'-bipyridine and, to a lesser extent, 2,3'-bipyridine and 2,4'-bipyridine , as well as nitrogen oxides and carbon monoxide . Pyridine is also classified as hazardous to health and class 2 water . In aquatic systems, pyridine damages both animal and plant organisms and is readily available due to its miscibility with water. The maximum permitted workplace concentration (MAK) in the DACH countries is 5 ppm.
toxicology
The contact with pyridine irritates the mucous membranes and the skin and disturbances of well- being occur , especially with regard to the gastrointestinal tract . Furthermore, pyridine has a low neurotoxic effect. A chronic exposure with pyridine may also disorders of the liver - and renal cause. In several test series, the genotoxicity and clastogenicity of pyridine could be excluded. The IARC classified pyridine as a possible carcinogen in 2017.
In most cases, pyridine is absorbed by inhalation , which leads to absorption in the lungs . The oral contrast uptake leads to absorption in the gastrointestinal tract. Pyridine is excreted either unchanged or metabolized in the faeces or urine . By metabolism occur as main products N -Methylpyryliumhydroxid represented by N -methyltransferases is formed, and the oxidation products pyridine N oxide and 2- , 3- and 4-hydroxypyridine , which by the action of monooxygenases arise on. However, humans metabolize pyridine exclusively to N -methylpyrylium hydroxide.
Ingestion of toxic doses of pyridine causes weakness, ataxia , salivation and can cause unconsciousness . From the year 1893 a death is known after accidental ingestion of half a cup of pyridine. The lowest known lethal dose (LD Lo ) for the oral intake of pyridine in humans is 500 mg kg −1 . Pyridine has a narcotic effect in higher concentrations and poses a serious health risk from a vapor concentration of 3600 ppm .
Pyridine in the environment
Small amounts of pyridine are released in industrial processes and given off into the environment. Traces of it are produced in steel production , coal gasification, coking plants, waste incineration and the processing of oil shale . In the ambient air of an oil shale processing plant, pyridine concentrations of up to 13 µg · m −3 or 53 µg · m −3 were found in the groundwater in the vicinity of a coal gasification plant. According to an investigation, 43,000 American workers are potentially in contact with pyridine.
proof
The UV / Vis spectrum of pyridine in hexane shows three absorption bands . These correspond to a π → π * transition at a wavelength of 195 nm ( extinction coefficient ε = 7500 l (mol cm) −1 ), another π → π * transition at 251 nm (ε = 2000 l ( mol cm) −1 ) and an n → π * transition at 270 nm (ε = 450 l (mol cm) −1 ).
In the 1 H- NMR spectrum of pyridine the protons show pronounced downfield shifts . The spectrum shows three signals corresponding to the three chemically different protons in the molecule. The signal integrals have a ratio of 2: 1: 2. The signal at the lowest field results from the α-protons δ (α-H) = 8.5 ppm, followed by the γ-proton δ (γ-H) = 7.5 ppm and the β-protons δ (β-H ) = 7.1 ppm. Benzene as a carbocyclic analog has a proton signal at δ = 7.27 ppm. The larger chemical shifts of the α- and γ-protons compared to benzene result from the lower electron density in the pyridine ring and correspond relatively to the lower electron densities in the α- and γ-positions, which can be derived from the mesomeric boundary structures. The chemical shifts of the 13 C nuclei behave analogously to the proton signals (δ (α-C) = 150 ppm, δ (β-C) = 124 ppm, δ (γ-C) = 136 ppm). In contrast, the 13 C signal of benzene is 129 ppm. All values refer to solvent-free substances.
Gas chromatographic or coupled gas and mass spectrometric methods are usually used for the quantitative determination of the pyridine concentration in environmental analysis .
Individual evidence
- ↑ a b c d e f g Entry on pyridine. In: Römpp Online . Georg Thieme Verlag, accessed on July 27, 2017.
- ↑ a b c d e f g h i j k l m n Entry on pyridine in the GESTIS substance database of the IFA , accessed on July 27, 2017(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Analytical Chemistry, pp. 8-44.
- ↑ a b c d David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-448.
- ↑ Entry on pyridines in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ MAK Documentation for Pyridine, 2009, doi : 10.1002 / 3527600418.mb11086d0047 (free full text)
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 110-86-1 or pyridine ), accessed on November 2, 2015.
- ↑ a b c d e f g h S. Shimizu, N. Watanabe, T. Kataoka, T. Shoji, N. Abe, S. Morishita, H. Ichimura: Pyridine and Pyridine Derivatives , in: Ullmann's Encyclopedia of Industrial Chemistry , 2005 , Wiley-VCH Weinheim.
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Thermochemistry, Electrochemistry, and Solution Chemistry, pp. 5-28.
- ^ A. Weissberger (Ed.), A. Klingsberg (Ed.), RA Barnes, F. Brody, PR Ruby: Pyridine and its Derivatives , Vol. 1, 1960 , Interscience Pub. Inc. New York.
- ↑ Th. V. Anderson: Products of the dry distillation of animal matter , in: Liebigs Ann. , 1849 , 70 , pp. 32-38; doi : 10.1002 / jlac.18490700105 .
- ↑ a b Th. Anderson: About the products of the dry distillation of animal matter , in: Liebigs Ann. , 1851 , 80 , pp. 44-65; doi : 10.1002 / jlac.18510800104 .
- ^ A. Ladenburg : Lectures on the history of the development of chemistry since the time of Lavoisier. English translation of a lecture. Full text access (PDF; 5.0 MB).
- ↑ About W. Ramsay's discovery in: Ber. German Chem. Ges. , 1877 , 10 , p. 736; doi : 10.1002 / cber.187701001202 .
- ↑ a b A. Tschitschibabin in: J. Prakt. Chem. , 1924 , 107 , p. 122.
- ^ A. Täufel, W. Ternes, L. Tunger, M. Zobel: Lebensmittel-Lexikon , 4th edition, p. 450, Behr Verlag, 2005, ISBN 3-89947-165-2 .
- ↑ GA Burdock (Ed.): Fenaroli's Handbook of Flavor Ingredients , Volume II, 3rd Edition, CRC Press, Boca Raton, 1995, ISBN 0-8493-2710-5 .
- ↑ J. Tang, QZ Jin, G.-H. Shen, C.-T. Ho, SS Cheng: Isolation and identification of volatile compounds from fried chicken , in: J. Agric. Food Chem. , 1983 , 31 , pp. 1287-1292; doi : 10.1021 / jf00120a035 .
- ↑ T. Shibamoto, Y. Kamiya, S. Mihara: Isolation and identification of volatile compounds in cooked meat: sukiyaki , in: J. Agric. Food Chem. , 1981 , 29 , pp. 57-63; doi : 10.1021 / jf00103a015 .
- ↑ C.-T. Ho, KN Lee, QZ Jin: Isolation and identification of volatile flavor compounds in fried bacon , in: J. Agric. Food Chem. , 1983 , 31 , pp. 336-342; doi : 10.1021 / jf00116a038 .
- ↑ J.-P. Dumont, J. Adda: Occurrence of sesquiterpenes in mountain cheese volatiles , in: J. Agric. Food Chem. , 1978 , 26 , pp. 364-367; doi : 10.1021 / jf60216a037 .
- ↑ HU Aeschbacher, U. Wolleb, J. Löliger, JC Spadone, R. Liardon: Contribution of coffee aroma constituents to the mutagenicity of coffee , in: Food Chem. Toxicol. , 1989 , 27 , pp. 227-231.
- ↑ O. Vitzthum, P. Factory Hoff, P. Hubert: New volatile constituents' of black tea aroma , in: J. Agric. Food Chem. , 1975 , 23 , pp. 999-1003; doi : 10.1021 / jf60201a032 .
- ^ A. Täufel, W. Ternes, L. Tunger, M. Zobel: Lebensmittel-Lexikon , 4th edition, p. 226, Behr Verlag, 2005, ISBN 3-89947-165-2 .
- ↑ M. Curvall, CR Enzell, B. Pettersson: An evaluation of the utility of four in vitro short term tests for predicting the cytotoxicity of individual compounds derived from tobacco smoke , in: Cell Biol. Toxicol. , 1984 , 1 , pp. 173-193; doi : 10.1007 / BF00125573 .
- ^ I. Schmeltz, D. Hoffmann: Nitrogen-containing compounds in tobacco and tobacco smoke , in: Chem. Rev. , 1977 , 77 , pp. 295-311; doi : 10.1021 / cr60307a001 .
- ↑ a b c d e f g h Study by the Occupational Safety & Health Administration, OSHA, Washington, DC, 1985. (PDF; 95 kB)
- ^ WH Powell: Revision of the extended Hantzsch-Widman System of nomenclature for heteromonocycles . in: Pure Appl. Chem. , 1983 , 55 , pp. 409-416, article (pdf; 187 kB) .
- ↑ D. Hellwinkel: The systematic nomenclature of organic chemistry , 4th edition, p. 45, Springer Verlag, Berlin, 1998, ISBN 3-540-63221-2 .
- ^ A. Gossauer: Structure and Reactivity of Biomolecules , 2006 , p. 488, Wiley-VCH Weinheim, ISBN 3-906390-29-2 .
- ↑ ICI DE-AS 1917037, 1968 .
- ↑ Nippon Kayaku, JP 7039545, 1967 .
- ↑ Koei Chemicals, BE 758201, 1969 .
- ↑ F. Mensch in: Erdöl Kohlen Erdgas Petrochemie , 1969 , 2 , pp. 67–71.
- ↑ A. Hantzsch : Condensation products from aldehyde ammonia and ketone-like compounds , in: Chem. Ber. , 1881 , 14 , pp. 1637-1638, doi : 10.1002 / cber.18810140214 .
- ↑ E. Knoevenagel , A. Fries: Syntheses in the pyridine series. About an extension of the Hantzsch dihydropyridine synthesis , in: Chem. Ber. , 1898 , pp. 761-767, doi : 10.1002 / cber.189803101157 .
- ↑ A. Behr: Applied homogeneous catalysis , 2008 , p. 722, Wiley-VCH Weinheim, ISBN 3-527-31666-3 .
- ↑ JB Tarr, J. Arditti: Niacin Biosynthesis in Seedlings of Zea mays , in: Plant Physiol. , 1982 , 69 , pp. 553-556; doi : 10.1104 / pp.69.3.553 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Fluid Properties, pp. 6-67.
- ↑ JP McCullough, DR Douslin, JF Messerly, IA Hossenlopp, TC Kincheloe, G. Waddington: Pyridine: experimental and calculated chemical thermodynamic properties between 0 and 1500 K., a revised vibrational assignment , in: J. Am. Chem. Soc. , 1957 , 79 , pp. 4289-4295; doi : 10.1021 / ja01573a014 .
- ^ A b V. Majer, V. Svoboda: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation , Blackwell Scientific Publications, Oxford, 1985, ISBN 0-632-01529-2 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-673.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Fluid Properties, pp. 6-211.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Fluid Properties, pp. 6-221.
- ↑ ES Domalski, ED Hearing: Heat Capacities and Entropies of Organic Compounds in the Condensed phase. Volume III , in: J. Phys. Chem. Ref. Data , 1996 , 25 , pp. 1-525; doi : 10.1063 / 1.555985 .
- ↑ D. Mootz, H.-G. Wussow: Crystal structures of pyridine and pyridine trihydrate , in: J. Chem. Phys. , 1981 , 75 , pp. 1517-1522; doi : 10.1063 / 1.442204 .
- ↑ Pyridine data sheet at AlfaAesar, accessed on June 26, 2010 ( PDF )(JavaScript required) .
- ↑ a b c d e J. A. Joules, K. Mills: Heterocyclic Chemistry , 5th Edition, pp. 125-141, Blackwell Publishing, Chichester, 2010, ISBN 978-1-4051-9365-8 .
- ↑ a b c d D. T. Davies: Basistexte Chemie: Aromatic Heterocyclen , 1st edition, Wiley-VCH, Weinheim 1995, ISBN 3-527-29289-6 .
- ^ R. Milcent, F. Chau: Chimie organique hétérocyclique: Structures fondamentales , 1st edition, pp. 241-282, EDP Sciences, 2002, ISBN 2-86883-583-X .
- ^ C. Elschenbroich : Organometallchemie , 6th edition, pp. 524-525, Vieweg + Teubner Verlag, 2008, ISBN 3-8351-0167-6 .
- ^ JA Joule, K. Mills: Heterocyclic Chemistry , 5th Edition, p. 7, Blackwell Publishing, Chichester, 2010, ISBN 1-4051-3300-7 .
- ^ C. Elschenbroich : Organometallchemie , 6th edition, p. 218, Vieweg + Teubner Verlag, 2008, ISBN 3-8351-0167-6 .
- ↑ a b c d e f g h i J. A. Joule, K. Mills: Heterocyclic Chemistry , 3rd Edition, 2004 , Blackwell Science, Oxford, ISBN 0-632-05453-0 .
- ↑ JM Bakke, I. Hegbom: Dinitrogen Pentoxide – Sulfur Dioxide, a New Nitration System , in: Acta Chemica Scandinavica , 1994 , 48 , pp. 181-182; doi : 10.3891 / acta.chem.scand.48-0181
- ↑ T. Murashima, K. Nishi, K.-I. Nakamoto, A. Kato, R. Tamai, H. Uno, N. Ono: Preparation of Novel Heteroisoindoles from Nitropyridines and Nitropyridones , in: Heterocycles , 2002 , 58 , pp. 301-310; doi : 10.3987 / COM-02-S (M) 22 .
- ↑ Joseph L. Duffy, Kenneth K. Laali: Aprotic Nitration (NO 2 + BF 4 - ) of 2-Halo- and 2,6-Dihalopyridines and Transfer-Nitration Chemistry of Their N -Nitropyridinium Cations , in: J. Org. Chem. , 1991 , 56 , pp. 3006-3009; doi : 10.1021 / jo00009a015 .
- ↑ O. Fischer : Note on nicotinic acid from pyridine , in: Chem. Ber. , 1882 , 15 , pp. 62-64; doi : 10.1002 / cber.188201501180 .
- ^ EF Möller, L. Birkofer: Constitutional specificity of nicotinic acid as a growth substance in Proteus vulgaris and Streptobacterium plantarum , in: Chem. Ber. , 1942 , 75 , pp. 1108-1118; doi : 10.1002 / cber.19420750912 .
- ↑ RN Shreve, EH Riechers, H. Rubenkoenig: Amination in the Heterocyclic Series by Sodium Amide , in: Ind. Eng. Chem. , 1940 , 32 , pp. 173-178; doi : 10.1021 / ie50362a008 .
- ^ GM Badger, WHF Sasse: The action of metal catalysts on pyridines , in: Adv. Heterocyc. Chem. , 1963 , 2 , pp. 179-202; doi : 10.1016 / S0065-2725 (08) 60749-7 .
- ^ WHF Sasse: 2,2'-bipyridine In: Organic Syntheses . 46, 1966, pp. 5-8, doi : 10.15227 / orgsyn.046.0005 ; Coll. Vol. 5, 1973, p. 102 ( PDF ).
- ↑ GH Burrows, LA King Jr .: The Free Energy Change that Accompanies Hydrogenation of Pyridine to Piperidine , in: J. Am. Chem. Soc. , 1935 , 57 , pp. 1789-1791; doi : 10.1021 / ja01313a011 .
- ↑ a b J.D. Cox, G. Pilcher: Thermochemistry of Organic and Organometallic Compounds , Academic Press, New York, 1970, pp. 1-636, ISBN 0-12-194350-X .
- ↑ DD Tanner, C.-M. Yang: On the structure and mechanism of formation of the Lansbury reagent, lithium tetrakis (N-dihydropyridyl) aluminate , in: J. Org. Chem. , 1993 , 58 , pp. 1840-1846; doi : 10.1021 / jo00059a041 .
- ↑ AJ De Koning, PHM Budzelaar, J. Boersma, GJM van der Kerk: Specific and selective reduction of aromatic nitrogen heterocycles with the bis-pyridine complexes of bis (1,4-dihydro-1-pyridyl) zinc and bis (1, 4-dihydro-1-pyridyl) magnesium , in: J. Organomet. Chem. , 1980 , 199 , pp. 153-170; doi : 10.1016 / S0022-328X (00) 83849-8 .
- ↑ M. Ferles: Coll. Czech. Chem. Comm. , 1959 , 24 , pp. 1029-1033.
- ↑ Report on the development of pyridine production in China.
- ^ A Leading Provider in a Variety of Markets. In: vertellus.com. Vertellus Specialties Inc., accessed March 18, 2016 .
- ^ EJ Corey, W. Suggs: Pyridinium Chlorochromate. An Efficient Reagent for Oxidation of Primary and Secondary Alcohols to Carbonyl Compounds , in: Tetrahedron Lett. , 1975 , 16 , pp. 2647-2650; doi : 10.1016 / S0040-4039 (00) 75204-X .
- ^ CH Bamford, CF H Tipper: Comprehensive Chemical Kinetics: Non-radical Polymerisation , 1st edition, Elsevier, Amsterdam, 1980, ISBN 0-444-41252-2 .
- ^ AV Hopper: Recent Developments in Polymer Research , 1st edition, Nova Science Publisher, 2007, ISBN 1-60021-346-4 .
- ^ RH Crabtree : Iridium compounds in catalysis , in: Acc. Chem. Res , 1979 , 12 , pp. 331-337; doi : 10.1021 / ar50141a005 .
- ^ Environmental Health Criteria (EHC) for Paraquat and Diquat , accessed November 19, 2014.
- ↑ K. Eller, E. Henkes, R. Rossbacher, H. Hoke: Amines, Aliphatic , in: Ullmann's Encyclopedia of Industrial Chemistry , 2005 , Wiley-VCH Weinheim.
- ^ CE Terry, RP Ryan, SS Leffingwell: Toxicology Desk Reference: The Toxic Exposure & Medical Monitoring Index: The Toxic Exposure and Medical Monitoring Index , 5th Edition, pp. 1062, Taylor & Francis, ISBN 1-56032-795-2 .
- ^ A. Täufel, W. Ternes, L. Tunger, M. Zobel: Lebensmittel-Lexikon , 4th edition, p. 218, Behr Verlag, 2005, ISBN 3-89947-165-2 .
- ↑ University of Jena: Water determination with Karl Fischer titration ( Memento from March 4, 2012 in the Internet Archive ).
- ↑ ECOTOX Database of the Environmental Protection Agency (EPA).
- ↑ Pyridine data sheet at AlfaAesar, accessed on June 3, 2010 ( PDF )(JavaScript required) .
- ↑ a b N. Bonnard, MT Brondeau, S. Miraval, F. Pillière, JC Protois, O. Schneider: Pyridine , Toxicological Data Sheet, INRS (French) .
- ↑ Yann Grosse, Dana Loomis, Kathryn Z Guyton, Fatiha El Ghissassi, Véronique Bouvard, Lamia Benbrahim-Tallaa, Heidi Mattock, Kurt Straif: Some chemicals that cause tumors of the urinary tract in rodents. In: The Lancet Oncology . 18, 2017, pp. 1003-1004, doi : 10.1016 / S1470-2045 (17) 30505-3 .
- ↑ G. Junk, C. Ford: A review of organic emissions from selected combustion processes , in: Chemosphere , 1980 , 9 , pp. 187-230; doi : 10.1016 / 0045-6535 (80) 90079-X .
- ↑ SB Hawthorne, R. E Sievers: Emissions of organic air pollutants from shale oil waste waters , in: Environ. Sci. Technol. , 1984 , 18 , pp. 483-490; doi : 10.1021 / es00124a016 .
- ↑ DH Stuermer, DJ Ng, CJ Morris: Organic contaminants in groundwater near an underground coal gasification site in northeastern Wyoming , in: Environ. Sci. Technol. , 1982 , 16 , pp. 582-587; doi : 10.1021 / es00103a009 .
- ↑ National Occupational Exposure Survey 1981–83, Cincinnati, OH, Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health.
literature
- E. Klingsberger: Pyridine - and Its Derivatives , 1st Edition, Interscience Publishers, New York, 1960, ISBN 0-470-37917-0 .
- T. Eicher, S. Hauptmann: The Chemistry of Heterocycles , 2nd edition, Wiley-VCH, Weinheim, 2003, ISBN 3-527-30720-6 .
- JA Joule, K. Mills: Heterocyclic Chemistry , 3rd Edition, Blackwell Science, Oxford, 2004, ISBN 0-632-05453-0 .
- DT Davies: Basistexte Chemie: Aromatic Heterocyclen , 1st edition, Wiley-VCH, Weinheim 1995, ISBN 3-527-29289-6 .