Nitronium tetrafluoroborate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
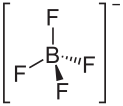
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Nitronium tetrafluoroborate | ||||||||||||||||||
| Molecular formula | NO 2 [BF 4 ] | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 132.81 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
240 ° C (decomposition) |
||||||||||||||||||
| solubility |
Decomposes in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
2.5 mg m −3 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Nitronium tetrafluoroborate is a chemical compound . It is the tetrafluoroborate - salt of the nitronium ion .
presentation
The salt can be made by reacting anhydrous hydrofluoric acid with boron trifluoride and nitrous pentoxide in nitromethane .
use
Nitronium tetrafluoroborate is used as a reactive reagent for the nitration of aromatics . Nitrations run better with this salt and under milder conditions than with the conventional nitration protocol, in which nitronium ions are produced in situ from nitric acid and sulfuric acid. It nitrates benzene to nitrobenzene and even 2,6-dibromopyridine .
Individual evidence
- ↑ a b c d data sheet nitronium tetrafluoroborate from AlfaAesar, accessed on March 2, 2010 ( PDF )(JavaScript required) .
- ↑ a b data sheet Nitronium tetrafluoroborate from Sigma-Aldrich , accessed on April 16, 2011 ( PDF ).
- ↑ K. Schofield: Aromatic nitration. 1st edition. Cambridge University Press, Cambridge, ISBN 0-521-23362-3 , p. 88.
- ↑ J. Buddrus: Fundamentals of organic chemistry. 3. Edition. de Gruyter Verlag, 2003, ISBN 3-11-014683-5 , p. 344.
- ↑ JL Duffy, KK Laali: Aprotic Nitration (NO 2 + BF 4 - ) of 2-Halo- and 2,6-Dihalopyridines and Transfer-Nitration Chemistry of Their N-Nitropyridinium Cations. In: J. Org. Chem. 1991, 56, pp. 3006-3009. doi : 10.1021 / jo00009a015

