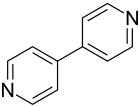
4,4'-bipyridine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 4,4'-bipyridine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 8 N 2 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 156.19 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
112 ° C . |
|||||||||||||||
| boiling point |
300-301 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
4,4'-Bipyridine is a heterocyclic chemical compound with the empirical formula C 10 H 8 N 2 . It consists of two pyridine rings that are linked to one another in the 4-position.
presentation
4,4'-bipyridine can be obtained in poor yield from the reaction of pyridine with lithium diisopropylamide and HMPT . Small amounts of 2,4'-bipyridine are formed as a by-product .
Another synthesis route consists of metal-mediated catalysis from 4-chloropyridine and pyridine or two molecules of 4-chloropyridine in the presence of a base on the nickel catalyst.
properties
At room temperature, it is a white to yellowish solid that melts at 112 ° C. and boiling at 300-301 ° C. The compound forms a dihydrate which melts incongruously at 67–70 ° C.
In the presence of metal ions , 4,4'-bipyridine can form polymeric complexes with these .
use
4,4'-bipyridine forms the basic structure of the herbicide paraquat ( N , N '-dimethyl-4,4'-bipyridinium dichloride), which can be produced from this.
Individual evidence
- ↑ a b c Kuhnert-Brandstätter, M .; Pröll, F .: Thermal analysis of hydrates of organic compounds. I in microchim. Acta 80 (1983) 463-476.
- ↑ Bhat, Aparna PI; Inam, Fawad; Bhat, Badekai Ramachandra: in Eur. J. Org. Chem. 31 (2013) 7139-7144.
- ↑ CR Smith: Skraup's reaction applied to Phenylenediamines. Preparation of the Phenanthrolines and related Dipyridyls , in: J. Am. Chem. Soc. , 1930 , 52 , pp. 397-403; doi : 10.1021 / ja01364a061 .
- ↑ a b Data sheet 4,4′-bipyridine (PDF) from Merck , accessed on December 18, 2019.
- ↑ GR Newkome, DC Hager: Interconversion of cembranolide δ- and γ-lactones: synthesis of the C-1 epimer of isolobophytolide , in: J. Org. Chem. , 1982 , 47 , pp. 599-601; doi : 10.1021 / jo00342a054 .
- ↑ R. Vanderesse, M. Lourak, Y. Fort, P. Careinigung: Activation of reducing agents. Sodium hydride containing complex reducing agents 23. Symmetrical coupling of nitrogen-containing heterocyclic halides , in: Tetrahedron Letters , 1986 , 27 , pp. 5483-5486; doi : 10.1016 / S0040-4039 (00) 85243-0 .
- ↑ Y. Fort, S. Becker, P. Ca Clean: A convenient synthetic route to bis-heteroaromatic and bis-heterocyclic compounds promoted by liganded nickel complex reducing agents. , in: Tetrahedron , 1994 , 50 , pp. 11893-11902.

