nickel
| properties | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Nickel, Ni, 28 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 10 , 4 , d | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | shiny, metallic, silvery | ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-02-0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-111-4 | ||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.283 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.015% | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 58.6934 (4) et al | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 135 (149) pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 124 pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 163 pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Ar ] 3 d 8 4 s 2 [Ar] 3d 9 4s 1 |
||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 7th.639 878 (17) eV ≈ 737.14 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 18th.168 838 (25) eV ≈ 1 753.03 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 35.187 (19) eV ≈ 3 395 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 54.92 (25) eV ≈ 5 299 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 76.06 (6) eV ≈ 7 339 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | Cubic area-centered | ||||||||||||||||||||||||||||||||||||||||||||||||
| density | 8.908 g / cm³ (20 ° C ) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 4.0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | ferromagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1728 K (1455 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 3003 K (2730 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 6.59 10 −6 m 3 mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 379 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 17.7 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 4970 m · s −1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 444 J kg −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Work function | 5.15 eV | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 13.9 · 10 6 A · V −1 · m −1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 91 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 2 , less often −1, 0, 1, 3, 4 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −0.257 V (Ni 2+ + 2 e - → Ni) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.91 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| MAK |
Switzerland: 0.5 mg m −3 (measured as inhalable dust ) |
||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicological data |
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||||||||
Nickel is a chemical element with the element symbol Ni and the atomic number 28. It is one of the transition metals , in the periodic table it is in the 8th subgroup or iron-platinum group according to the older counting method , and according to the newer in group 10 or nickel group .
history

Nickel was first presented in pure form by Axel Frederic Cronstedt in 1751 and named after the mineral Kupfernickel ( Swedish kopparnickel , today Nickelin ) in which he found the previously unknown metal.
The medieval miners used the name Kupfernickel for the ore , which looked like copper ore , but from which no copper could be extracted, as if it had been bewitched by mountain spirits ("nickels"). A similar goblin-like etymology can be found in cobalt .
The first pure nickel coin was minted in 1881.
Occurrence
Nickel occurs in the earth's crust with a content of about 0.008%. Based on geophysical and geochemical evidence, it is believed that most of the nickel on Earth and other terrestrial planets is in the core, where it forms an alloy with iron and some light elements. According to the latest models, its mass fraction in the earth's core is around 5.2%.
Solid , i.e. nickel only rarely occurs in its elemental form. So far, only around 50 sites for native nickel have been documented (as of 2018), including in Australia, China, Canada, Russia and the United States of America.
Traditionally, most of the nickel production is made from sulphidic ores such as pentlandite (approx. 34% nickel), magnetic nickel gravel (aggregate of pyrrhotite and pentlandite) and some other nickel minerals such as millerite (approx. 64–65% nickel) and nickel linseed (approx. 44%) Nickel). In addition, lateritic nickel ores, mainly made from garnierite , a mixture of népouite (approx. 46% nickel) and willemseit (approx. 29% nickel), are mined as raw materials for nickel production. A total of around 200 nickel minerals are known to date, and some have far higher nickel contents than those already mentioned, but in contrast to these are much less common. For example, the very rare bunsenite is the mineral with the highest nickel content of up to 78.58%. The also rare minerals Heazlewoodite and Awaruit contain between 72 and 73% nickel.
The extraction is increasingly shifting to lateritic nickel ores due to the exploitation of the classic sulphidic deposits. These must, however, consuming (by high pressure acid leaching English high pressure acid leaching be won).
In order to be able to mine nickel economically, the nickel content of the ore must be at least 0.5%. The most important occurrences are found in Canada ( Sudbury Basin ), New Caledonia , Russia ( Norilsk and Kola Peninsula ), Australia ( Queensland ) and Cuba ( Moa Bay and Nicaro ). A frequent companion of nickel is cobalt .

| rank | country | Production (in million t ) |
|---|---|---|
| 1 | Indonesia | 400,000 |
| 2 | Philippines | 230,000 |
| 3 | New Caledonia | 210,000 |
| 4th | Canada | 210,000 |
| 5 | Australia | 190,000 |
| 6th | Russia | 180,000 |
| 7th | Brazil | 140,000 |
| 8th | People's Republic of China | 98,000 |
| 9 | Guatemala | 68,000 |
| 10 | Cuba | 51,000 |
Nickel as a mineral
Naturally occurring nickel in its elemental form was first described by Paul Ramdohr in 1967 and recognized by the International Mineralogical Association (IMA) as an independent mineral type (IMA's internal entry number: 1966-039 ).
According to the systematics of minerals according to Strunz (9th edition) , nickel is classified under system no. 1.AA.05 (elements - metals and intermetallic compounds - copper cupalite family - copper group) or in the outdated 8th edition classified under I / A.04b ( nickel series ). The systematics of minerals according to Dana , which is mainly used in English-speaking countries , lists the element mineral under the system no. 01.01.11.05 ( iron-nickel group ).
The type locality is the Bogota peninsula near Canala in the northern province of New Caledonia , where native nickel was found in the form of idiomorphic cubic grains or ingrown cubes up to about 0.1 mm as inclusions in Heazlewoodite and as a "spider-like" irregular mass between the Heazlewoodite grains . In addition to Heazlewoodite, accompanying minerals may include chalcopyrite , chalcosine , galena , godlevskite , solid copper , millerite , orcelite , pentlandite , pyrite and pyrrhotite .
Extraction and presentation
Representation of the copper-nickel fine stone
The majority of nickel is obtained from iron ores containing nickel and copper, such as magnetic nickel gravel . In order to make extraction economical, the nickel must first be enriched to a nickel content of around five percent by flotation . The ore is then roasted in a similar way to copper production . The ore is first roasted in order to convert part of the iron sulfide into iron oxide . Then, silicates and coke added to the iron oxide as iron silicate to slagged . At the same time, the raw copper-nickel stone is formed from nickel, copper and iron sulfide. Since this is specifically heavier than the iron silicate slag, the two phases can be tapped separately.
The raw stone is then placed in a converter and silicon dioxide is added. It is oxygen blown. As a result, the remaining iron sulfide is roasted into iron oxide and then slagged. The result is copper-nickel fine stone , which consists of around 80% copper and nickel and around 20% sulfur.
Extraction of raw nickel
In order to obtain the raw nickel, the nickel must be separated from the copper. To do this, the fine stone is fused with sodium sulphide Na 2 S. A slightly melting double sulphide is only formed between copper and sodium sulphide. Two easy-to-separate phases of copper-sodium double sulfide (liquid) and nickel sulfide are formed. After the separation, the nickel sulfide is roasted to nickel oxide and then reduced to nickel with coke.

Extraction of pure and pure nickel

In order to obtain pure nickel, the raw nickel is electrolytically refined. For this purpose, the raw nickel is connected as the anode and a thin sheet of nickel as the cathode in an electrolysis cell . A nickel salt solution is used as the electrolyte . During the electrolysis, nickel and all the less noble components dissolve at the anode. All the more noble components remain solid and fall under the electrode as anode sludge . This serves as an important source for the production of precious metals such as gold or platinum . At the cathode, nickel ions are reduced from the solution to nickel, all less noble components remain in solution. The purity of electrolyte nickel is around 99.9%.
For the extraction of pure nickel with a purity of 99.99% there is a special process called the moon process , named after Ludwig Mond , who discovered nickel tetracarbonyl in 1890 . This process is based on the formation and decomposition of nickel tetracarbonyl. For this purpose, finely divided raw nickel powder is placed in a stream of carbon monoxide at 80 ° C. Gaseous nickel tetracarbonyl is thereby formed. This is freed from fly dust and fed into a decomposition chamber at 180 ° C. There are small nickel balls inside. On these, the nickel tetracarbonyl decomposes again to nickel and carbon monoxide. This results in very pure nickel.
Laboratory display
There are different methods for representing small amounts of very pure nickel in the laboratory:
- Reduction of the oxide with hydrogen at 150 ° C to 250 ° C:
- Reduction of a nickel (II) chloride suspension in diethyl ether via a Grignard reaction
- Thermal decomposition of nickel (II) oxalate in the absence of oxygen:
- Reduction of nickel (II) chloride with a sodium dispersion:
In particular, the thermolysis of the oxalate produces finely divided pyrophoric nickel powder.
properties
Physical Properties
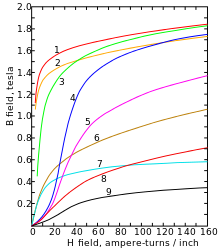
1. sheet steel , 2. electrical sheet , 3. Cast steel , 4. Tungsten steel ,
5. Magnetic steel , 6. Cast iron , 7. Nickel, 8. Cobalt , 9. Magnetite

Nickel is a silvery-white metal which, with a density of 8.91 g / cm³, is one of the heavy metals . It is medium hard ( Mohs hardness 3.8), malleable, ductile and can be polished excellently. Like iron and cobalt , nickel is ferromagnetic , with a Curie temperature of 354 ° C. The metal crystallizes in a face-centered cubic crystal structure ( copper type) in the space group Fm 3 m (space group no. 225) with the lattice parameter a = 352.4 pm and four formula units per unit cell . It maintains this structure even at high pressures up to at least 70 GPa. Another metastable modification with body-centered cubic spherical packing could be obtained in thin layers on iron or gallium arsenide . At 183 ° C, it has a significantly lower Curie temperature.
The tensile strength of soft-annealed nickel is 400–450 MPa with an elongation at break between 30 and 45%. The hardness values are around 80 HB. Cold-hardened nickel, whose elongation at break is below 2%, achieves strengths of up to 750 MPa with hardness values around 180 HB. Pure nickel semi-finished products with 99% Ni content can be highly hardened when cold.
The isotope 62 Ni has the highest binding energy per nucleon of all isotopes of all elements .
Chemical properties
Nickel is very resistant to air, water, hydrochloric acid and alkalis at room temperature. Dilute acids attack nickel only very slowly. In contrast to concentrated oxidizing acids ( nitric acid ), passivation occurs analogously to stainless steel . Nickel is soluble in dilute nitric acid (approx. 10 to 15 percent). Half-concentrated nitric acid (approx. 30 percent) also causes noticeable passivation. The most common oxidation state is + II, less often −I, 0, + I, + III and + IV are observed. In nickel tetracarbonyl , nickel has the oxidation number 0. Nickel (II) salts dissolve in water with the formation of aqua complexes with a greenish color.
Finely divided nickel reacts with carbon monoxide at 50 to 80 ° C to form nickel tetracarbonyl , Ni (CO) 4 , a colorless, very toxic liquid. This serves as an intermediate product for the production of the purest nickel using the moon process . At 180 to 200 ° C, nickel tetracarbonyl breaks down again into nickel and carbon monoxide.
physiology
The controversial essentiality of nickel contrasts with the existence of several enzymes that normally contain nickel, but do not depend on it, since its role as a cation can be taken over by other divalent cations. In humans, these are three proteins that are known to bind nickel:
- Alpha-fetoprotein binds nickel, but does not depend on it as it is not an enzyme
- Acireductone Dioxygenase , an enzyme from the methionine salvage pathway that usually binds nickel or another divalent cation
- Polyribonucleotide 5'-hydroxylkinase Clp1 , which requires magnesium, manganese or nickel as a cofactor
For plants and various microorganisms, the essentiality of nickel is determined by the isolation of several enzymes (e.g. urease , Co-F430 ) that contain nickel in the active center, as well as by the detection of deficiency symptoms in a nickel-poor environment, which can be increased by adding Ni (II ) -Salts removed, secured.
In electrophysiology , nickel ions are used to block voltage-activated calcium channels .
Health problems
Together with nickel dermatitis, nickel is the most common cause of contact allergies : an estimated 1.9 to 4.5 million people in Germany are sensitized to nickel. Due to changes in legislation, metals and alloys that come into contact with the skin are less often nickel-plated. About 10% of all children are sensitized to nickel. If they come into contact with the allergen again , they can react with a contact allergy. In addition, an increased nickel content in the air we breathe and in drinking water is a risk factor for sensitization to nickel in children.
The tolerable daily intake (TDI) of nickel amounts to according to European Food Safety Authority (EFSA) 2.8 micrograms (0.0028 mg) per kilogram body weight. In 2019, the Upper Austrian Chamber of Labor had twelve different soy drinks examined by the Agency for Health and Food Safety. The values were between 0.25 ( Dennree Soy Drink Natural) and 0.69 milligrams per liter ( Yes! Of course, organic soy drink). In the case of the soy milk with the highest values, a child weighing 30 kg has already consumed more than twice as much nickel as recommended by the EFSA with a quarter liter.
Inhalation of inorganic nickel compounds is associated with an increased risk of squamous cell carcinoma of the lung and upper airways. Such malignant neoplasms are recognized as occupational diseases in Germany in the event of occupational exposure (BK 4109).
Prohibitions
In order to contain the risks of being sensitive to nickel and acting as an allergen , in 1994 the European Union demanded regulations from its member states to limit the use and placing on the market of products made from nickel or nickel compounds that come into direct and prolonged contact with human skin, such as ear studs, watch cases, Necklaces, spectacle frames or zippers as part of clothing; The limit value determined was a nickel release (nickel release ) of more than 0.5 micrograms per square centimeter and week, which, even with a coated product made of nickel, could not be exceeded after two years of use. In Germany, these bans were implemented by the Consumer Goods Ordinance for consumer goods , i.e. items with skin contact or with food, and were later supplemented by labeling requirements. The use of nickel and the placing on the market of such products has been directly and effectively regulated in the European Union since 2009. Accordingly, the limit ( migration limit ) of 0.5 μg / cm² / week still applies , but now 0.2 μg for sticks that are pierced into the skin (ear studs, piercings). In practice, however, it can be unclear, for example, when assessing toys, sewing needles or pens what “longer” skin contact actually means.
use
Nickel is required as a metal in small quantities; most of the production goes into the production of stainless steels and nickel alloys . Nickel is used in many specific and recognizable industrial and consumer uses, including steel , Alnico - magnet , coins , rechargeable batteries , e- guitar strings , microphone capsules , claddings on sanitary fittings and special alloys such as permalloy , Elinvar and Invar . It is used for coating and as a green tint in glass . The reserves of nickel deposits that are mineable from today's point of view are between 70 and 170 million tons. At present, well over a million tonnes (2006: 1.340 million tonnes) are extracted worldwide every year. Due to financial market speculation, the price of nickel is subject to very high price fluctuations at times.
Around 25 percent of the world's nickel deposits are in New Caledonia , a French overseas territory .
Use as metal
Pure nickel metal is used in finely divided form as a catalyst in the hydrogenation of unsaturated fatty acids . Due to its chemical resistance , nickel is used for apparatus in chemical laboratories and the chemical industry (e.g. nickel crucibles for digestions ). Nickel alloys , e.g. B. for coins .
Nickel is used as a coating metal for corrosion protection ("nickel plating") of metal objects: Because of its protective properties against oxidation , metals (especially iron ) are coated with a nickel layer for certain technical purposes using galvanic technology .
The metal was also used earlier to manufacture the frames of nickel glasses .
The isotopic 63 Ni nickel is used as a beta emitter in electron capture detectors in gas chromatographs .
Use as an alloy
Nickel is an important alloy metal that is mainly used for steel finishing . Most of the nickel goes there. It makes steel resistant to corrosion and increases its hardness , toughness and ductility . Steels high-alloyed with nickel are used in particularly corrosive environments. The stainless steel V2A (the name comes from the "test batch 2 austenitic " in the Krupp steelworks, corresponds to X12CrNi18-8) contains 8% nickel in addition to 18% chromium , V4A (brand names Cromargan or Nirosta ) 11% in addition to 18% chromium and 2% molybdenum .
Nickel is an excellent alloying agent for certain precious metals and is used in the fire test as a collector of elements of the platinum metals . As such, nickel is able to collect all six platinum metals, especially platinum and palladium , entirely from ores and partially collect gold .
Nickel foam or nickel mesh is used in gas diffusion electrodes for alkaline fuel cells .
Nickel and its alloys are widely used as catalysts for hydrogenation reactions. Raney nickel , a finely divided nickel- aluminum alloy, is a common form, although related catalysts are also used, including Raney-type catalysts.
About 20% of nickel is used (in Germany) for the production of other nickel alloys :
- Constantan , an alloy of 55% copper and 45% nickel, which has an almost constant specific electrical resistance over a wide temperature range . It is mainly used for precise resistances .
- Nickel-based superalloys are alloys specially designed for use at high temperatures and under corrosive media. They are used, for example, in aircraft turbines and gas turbines in power plants .
- Raney nickel , a nickel- aluminum - alloy , which is an important catalyst for the hydrogenation is of organic compounds.
- Nickel silver , a copper -Nickel- zinc - alloy with 10-26% nickel content, which is particularly resistant to corrosion and is mainly used for cutlery and electrical is used devices.
- Monel , also a copper- nickel alloy with around 65% nickel, 33% copper and 2% iron , which is characterized by particular chemical resistance , including fluorine . It is therefore used for fluorine pressurized gas cylinders .
- Austenitic cast iron with spheroidal graphite , a spherulitic special cast iron with up to 20% nickel, for use in corrosive environments and at high temperatures .
proof
The detection reaction for the nickel (II) salts, which are usually green in water, is carried out gravimetrically in quantitative analysis and qualitatively in the cation separation process with dimethylglyoxime solution (Tschugajew's reagent). Nickel salts are previously precipitated as gray-black nickel (II) sulfide using ammonium sulfide, if necessary, and dissolved in nitric acid. The specific detection is then possible by reaction with dimethylglyoxime in an ammoniacal solution. The raspberry-red bis (dimethylglyoximato) nickel (II) precipitates as a complex :
.
Since nickel precipitates quantitatively from ammoniacal solution with dimethylglyoxime, this proof can also be used for quantitative gravimetric nickel analysis. A quantitative determination can also be carried out from ammoniacal solution using electrogravimetry on a platinum mesh electrode. Similar to other heavy metals, nickel is now mostly determined quantitatively by atomic spectroscopy or mass spectrometry, also in the ultra-trace range. Inverse voltammetry with adsorptive accumulation of the Ni-dimethyglyoxime complex on hanging mercury droplets or mercury film electrodes is extremely sensitive .
links
Nickel is found in compounds mainly in the + II oxidation state . Levels 0, + I, + III and + IV are rare and mostly unstable . Nickel forms a large number of mostly colored complexes .
Oxides
Nickel (II) oxide and nickel (III) oxide are green and black solids, respectively, and are used in the manufacture of ceramics , glasses and electrodes . They are also used as catalysts for the hydrogenation of organic compounds . Like many other binary metal oxides, nickel (II) oxide is often not stoichiometric , which means that the nickel-to- oxygen ratio deviates from 1: 1. This property is accompanied by a color change, the stoichiometrically correct nickel (II) oxide being green and the non-stoichiometric nickel (II) oxide being black. Nickel (III) oxide has a strong oxidizing effect and is unknown as a pure substance .
Halides
Nickel (II) chloride is a yellow, strongly hygroscopic solid that is used as a dye for ceramics and for the production of nickel catalysts. In addition to the anhydrous form, there are also hydrous nickel (II) chlorides, e.g. B. the green nickel (II) chloride hexahydrate , which crystallizes from aqueous nickel chloride solutions. The anhydrous nickel (II) chloride has a trigonal crystal structure of the cadmium (II) chloride type with the space group R 3 m (space group no. 166) . The hexahydrate crystallizes in the monoclinic crystal system in the space group C 2 / m (space group no. 12) .
Nickel (II) fluoride is also very hygroscopic and forms yellowish to green tetragonal crystals . In contrast to many fluorides , it is stable in air. It crystallizes in the tetragonal crystal system with the space group P 4 2 / mnm (space group no. 136) . The tetrahydrate crystallizes in the orthorhombic crystal system with the space group P 2 1 from (space group no. 29, position 3) .
Other inorganic nickel compounds
Nickel (II) hydroxide and nickel (III) oxide hydroxide are used to store electrical energy in nickel, cadmium and other nickel accumulators .
Nickel (II) nitrate is used in the ceramics industry as a brown pigment , in dyeing as a pickling agent , for electrolytic nickel plating , for obtaining nickel (II) oxide and for producing pure catalyst nickel . Nickel (II) nitrate is a strong oxidizing agent and usually occurs in the form of its hexahydrate Ni (NO 3 ) 2 · 6H 2 O.
Nickel (II) sulfate and ammonium nickel (II) sulfate are used in electroplating ( nickel plating ). Nickel (II) sulfate is the technically most important nickel compound. It is used to produce other nickel compounds and catalysts . The aqueous solutions of nickel (II) sulfate and nickel (II) chloride are used for the electrodeposition of metallic nickel layers. It is also used in dyeing as a mordant and in the manufacture of gas masks .
Nickel (II) carbonate occurs in several hydrate forms. It is used as a catalyst in fat hardening and in the production of nickel (II) oxide, ceramic colors (pigments) and glazes as well as in electroplating . It formed a trigonal crystal system with the space group R 3 c (space group no. 161) .
Nickel (II) sulfide precipitates from ammoniacal , but not from acidic , nickel-containing solutions with ammonium sulfide . This allows nickel with the ammonium sulfide group to be separated in the cation separation process.
Nickel antimonide, known as Breithauptit, is a shiny metallic mineral and has a bright copper-red color. Nickel antimonide is used as a material in magnetic field plates where it is placed between magnetically sensitive layers of indium antimonide . Magnetic field plates change their electrical resistance depending on the magnetic flux density and serve as a sensor for magnetic fields . It forms a hexagonal crystal structure in the space group P 6 3 / mmc (space group no. 194) .
Organic nickel compounds
Nickel tetracarbonyl Ni (CO) 4 is a colorless, very toxic liquid . It is an important intermediate product in the lunar process . Nickel tetracarbonyl was the first metal carbonyl compound discovered .
Nickel complexes
Nickel and v. a. Nickel (II) ions form many, mostly colored complexes . The coordination numbers 6, 5, or 4 are the most common. In the case of weak, monodentate ligands , such as water , they are usually present as octahedral and paramagnetic high-spin complexes with coordination number 6. Strong ligands such as cyanide form square-planar, diamagnetic low-spin complexes. Dimethylglyoxime also forms a square-planar complex , as the complex is additionally stabilized by hydrogen bonds. The latter bis (dimethylglyoximato) nickel (II) complex is important for the wet chemical detection of nickel. Anionic nickel complexes end in "-niccolate".
Examples of ammine complexes are the blue tetraammine nickel (II) and purple hexaammine nickel (II) complex. Both compounds are obtained by adding ammonia to nickel (II) salt solutions:
By adding potassium cyanide to nickel (II) salt solutions, initially nickel (II) cyanide is formed , which in excess of potassium cyanide dissolves to yellow potassium tetracyanonic colate (II):
A corresponding compound is formed with potassium thiocyanate. Potassium hexafluoroniccolate (IV) (K 2 [NiF 6 ]) is a very sensitive compound . With a strong reducing agent, the binuclear complex K 4 [Ni 2 (CN) 6 ] with monovalent nickel can be produced from potassium tetracyanonic colate (II) . In addition, there are a number of complexes with organic ligands such as ethylene diamine or anions of carboxylic acids .
literature
- Eberhard Auer, Siegfried Müller, Rainer Slotta : 250 years of nickel. From "nickel" to "EURO". Verlag Deutsches Bergbau-Museum, series of publications from the German Mining Museum Bochum Vol. 95, Bochum 2001, ISBN 3-921533-81-3 .
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1709-1721.
- Harry H. Binder: Lexicon of the chemical elements - the periodic table in facts, figures and data. Hirzel, Stuttgart 1999, ISBN 3-7776-0736-3 .
Web links
- Mineral Atlas: Nickel (Wiki)
- Pure nickel> = 99.9% as a picture in Heinrich Pniok's collection
- Nickel prices as a diagram (English)
- Medical text about nickel deficiency or excess in the body by eesom AG, Bern (CH).
Individual evidence
- ↑ Scerri, Eric R .: The periodic table: its story and its significance . Oxford University Press, 2007, ISBN 0-19-530573-6 , pp. 239-240.
- ^ Harry H. Binder: Lexicon of the chemical elements , S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (nickel) , unless otherwise stated .
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e Entry on nickel in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e Entry on nickel at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ NN Greenwood and A. Earnshaw: Chemistry of the elements , 1st edition, VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 1469.
- ↑ a b c Derek GE Kerfoot: Nickel in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2005, doi : 10.1002 / 14356007.a17_157
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data. 56, 2011, pp. 328-337, doi : 10.1021 / je1011086 .
- ↑ Ruhr University Bochum, Institute for Materials: Nickel ( Memento of the original from September 24, 2015 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ Ludwig Bergmann, Clemens Schaefer, Rainer Kassing: Textbook of Experimental Physics , Volume 6: Solid . 2nd edition, Walter de Gruyter, 2005, ISBN 978-3-11-017485-4 , p. 361.
- ↑ a b c d e Entry on nickel, powder in the GESTIS substance database of the IFA , accessed on April 18, 2020(JavaScript required) .
- ↑ Entry on nickel in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for 7440-02-0 or nickel, metal ), accessed on November 2, 2015.
- ↑ Hans Lüschen: The names of the stones. The mineral kingdom in the mirror of language . 2nd Edition. Ott Verlag, Thun 1979, ISBN 3-7225-6265-1 , p. 104, 260 .
- ↑ List of elements and their abundance in the earth's crust at uniterra.de
- ^ William F. McDonough: Compositional Model for the Earth's Core . In: Richard W. Carlson (Ed.): Treatise on Geochemistry . tape 2 . Elsevier, 2014, p. 559–577 , doi : 10.1016 / B0-08-043751-6 / 02015-6 ( mso.anu.edu.au [PDF; 621 kB ; accessed on February 7, 2019]).
- ↑ Find location list for nickel in the Mineralienatlas and Mindat
- ↑ Martin Okrusch, Siegfried Matthes: Mineralogie. An introduction to special mineralogy, petrology and geology . 7th, completely revised and updated edition. Springer, Berlin [a. a.] 2005, ISBN 3-540-23812-3 , pp. 37, 242 .
- ↑ Webmineral - Mineral Species containing Nickel (Ni) (English).
- ↑ Gavin M. Mudd, 2009, Nickel Sulfide Versus Laterite: The Hard Sustainability Challenge Remains . Proc. “48th Annual Conference of Metallurgists,” Canadian Metallurgical Society, Sudbury, Ontario, Canada, August 2009.
- ↑ Xue-yi GUO, Wen-tang SHI, Dong LI, Qing-hua TIAN: Leaching behavior of metals from limonitic laterite ore by high pressure acid leaching . In: Transactions of Nonferrous Metals Society of China . tape 21 , no. 1 , January 2011, p. 191 , doi : 10.1016 / S1003-6326 (11) 60698-5 (English).
- ^ A b Michael Fleischer : New Mineral Names . In: American Mineralogist . tape 53 , 1968, pp. 348–351 ( minsocam.org [PDF; 295 kB ; accessed on January 26, 2018]).
- ^ IMA / CNMNC List of Mineral Names; July 2019 (PDF 1.67 MB; nickel see p. 138)
- ↑ IMA / CNMNC List of Mineral Names 2009 (English, PDF 1.8 MB, Nickel p. 202)
- ↑ Webmineral - Minerals Arranged by the New Dana classification. 01/01/11 Iron-Nickel group
- ↑ Nickel . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 (English, handbookofmineralogy.org [PDF; 57 kB ; accessed on February 7, 2019]).
- ↑ TP Whaley: Nickel powder . In: Therald Moeller (Ed.): Inorganic Syntheses . tape 5 . McGraw-Hill, Inc., 1957, pp. 195-197 (English).
- ^ Charles Steinmetz: Theory and Calculation of Electric Circuits . Editor: McGraw-Hill. 1917, Fig. 42 .
- ^ A b Ying Zhu, Ping Yu, Xiaofeng Jin, Ding-sheng Wang: Curie temperature of body-centered-tetragonal Ni. In: Journal of Magnetism and Magnetic Materials. 2007, 310, 2, pp. E301-e303, doi : 10.1016 / y.jmmm.2006.10.240 .
- ↑ K. Schubert: A model for the crystal structures of the chemical elements . In: Acta Crystallographica . 1974, B30, pp. 193-204, doi : 10.1107 / S0567740874002469 .
- ^ NB Brookes, A. Clarke, PD Johnson: Electronic and magnetic structure of bcc nickel. In: Phys. Rev. B. 1992, 46, pp. 237-241, doi : 10.1103 / PhysRevB.46.237 .
- ↑ MP Fewell: The atomic nuclide with the highest mean binding energy . In: American Journal of Physics . 63, No. 7, 1995, pp. 653-658. bibcode : 1995AmJPh..63..653F . doi : 10.1119 / 1.17828 .
- ↑ Search result UniProt Nickel / Human .
- ↑ UniProt Q9BV57
- ↑ UniProt Q92989
- ↑ A. Schnuch, W. Uter, J. Geier, O. Gefeller: Epidemiology of contact allergy. An estimation of morbidity employing the clinical epidemiology and drug-utilization research (CE-DUR) approach. In: Contact Dermatitis 47 (1), 2002, pp. 32-39; PMID 12225411 .
- ↑ BfR : Contact allergens in toys: Health assessment of nickel and fragrances (PDF; 178 kB), updated BfR opinion No. 010/2012 of April 11, 2012.
- ↑ Prevalence of nickel sensitization and urinary nickel content of children are increased by nickel in ambient air; PMID 21168833 .
- ↑ Allergenic potential: nickel in drinking water .
- ↑ Soy drinks: Alarmingly high nickel contents. In: ooe.arbeiterkammer.at. Retrieved May 28, 2019 .
- ↑ Jürgen Strutz, Olaf Arndt, Wolfgang Mann: Practice of ENT medicine, head and neck surgery. Thieme, 2001, ISBN 3-13-116971-0 , p. 386.
- ↑ Directive 94/27 / EC of the European Parliament and of the Council of June 30, 1994 , the so-called Nickel Directive
- ↑ When BedGgstV 1992 came into force, only ear studs and Ä. That remain in the wound canal until epithelialization is a prohibited object according to § 3, appendix. 1 no. 6. Obligations for labeling followed in Section 10 (6), Annex. 9. Currently (as of January 2020) the maximum values of nickel release for commercial placing on the market in accordance with Annex 5a to Section 6 BedGgstV are still valid . Infringements against this are punishable according to § 12 Abs. 3 in connection with § 59 LFGB . Bavarian State Office for Health and Food Safety : Nickel in consumer goods ( Memento from March 31, 2016 in the Internet Archive )
- ↑ Annex XVII No. 27 to Article 67 of Regulation (EC) No. 1907/2006 of the European Parliament and of the Council , also known as the REACH Regulation .
- ↑ Magdalena Köhler, ballpoint pens do not only come into temporary contact with the body , Chemical and Veterinary Investigation Office Stuttgart, July 8, 2016. German Association of the Toy Industry i. V., member information on the arguments against the market surveillance offices from August 4, 2015. On practice and especially on tattoo inks: Peter Laux: Schmuck & Co, Nickel is everywhere ... , Federal Institute for Risk Assessment , 2014
- ↑ American Plumbing Practice: From the Engineering Record (Prior to 1887 the Sanitary Engineer.) A Selected Reprint of Articles Describing Notable Plumbing Installations in the United States, and Questions and Answers on Problems Arising in Plumbing and House Draining. With Five Hundred and Thirty-six Illustrations . Engineering record, 1896, p. 119. Archived from the original on December 1, 2016 (Retrieved May 28, 2016).
- ↑ Handelsblatt: Nickel price breaks records. April 10, 2007. ( Memento of the original from March 31, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Rohstoff-Welt.de »Analyzes» LME-Nickel marks new annual low, volatility has risen sharply .
- ↑ Kharton, Vladislav V .: Solid State Electrochemistry II: Electrodes, Interfaces and Ceramic Membranes . Wiley-VCH, 2011, ISBN 978-3-527-32638-9 , pp. 166-. Archived from the original on September 10, 2015 (Retrieved June 27, 2015).
- ↑ Bidault, F .: A New Cathode Design for Alkaline Fuel Cells (AFCs) . Imperial College London. Archived from the original on July 20, 2011.
- ↑ E. Schweda: Jander / Blasius: Inorganic Chemistry II - Quantitative Analysis & Preparations . 16th edition. Hirzel, 2012, ISBN 978-3-7776-2133-3 , pp. 87 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1715.
- ^ AF Wells: Structural Inorganic Chemistry , Oxford Press, Oxford , United Kingdom , 1984.
- ↑ J. Mizuno: The Crystal Structure of Nickel Chloride Hexahydrate, NiCl 2 · 6 H 2 O , in: Journal of the Physical Society of Japan , 1961, 16 (8), p. 1574. bibcode : 1961JPSJ ... 16.1574M .
- ^ Jean D'Ans, Ellen Lax: Pocket book for chemists and physicists. 3. Elements, inorganic compounds and materials, minerals, Volume 3. 4. Edition, Springer, 1997, ISBN 978-3-540-60035-0 , p. 640 ( limited preview in Google book search).
- ↑ Entry on nickel (II) sulfate. In: Römpp Online . Georg Thieme Verlag, accessed on January 5, 2015.
- ↑ Entry on nickel (II) carbonate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Georg Brauer : Handbook of Preparative Inorganic Chemistry.
- ^ Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 85 .
- ↑ Wolfgang Glöckner: The complex connections, Aulis Verlag Cologne 1962, pp. 103-107.





![{\ displaystyle {\ ce {NiC2O4 -> [T] [] Ni + 2 CO2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4f5ac7119d30ce593cf99675097e91e39e33a04d)











![{\ displaystyle {\ ce {NiSO4 + 4NH4 + + 4OH- -> [Ni (NH3) 4] SO4 + 4H2O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fed711e628c3ba92046eb178a459488074a035c9)
![{\ displaystyle {\ ce {Ni (CN) 2 + 2KCN -> K2 [Ni (CN) 4]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7a2b8181f20f5d9ad99c2900689b699739283d0d)