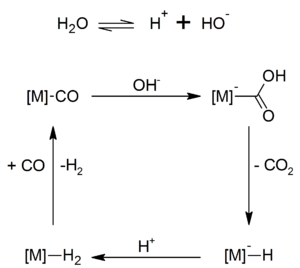Metal carbonyls
Metal carbonyls are complex compounds of transition metals with carbon monoxide as a ligand . The metals occur in these compounds with an oxidation number of ± 0 and their properties sometimes differ considerably from the classic metal compounds. Mononuclear metal carbonyls with only one central atom are often readily soluble in organic solvents and highly volatile. The carbon monoxide ligand can be replaced by a large number of other ligands, thus leading to mixed metal carbonyls.
The compounds are used in organic synthesis and as catalysts or catalyst precursors in the chemical industry , for example in hydroformylation and Reppe chemistry . In the Mond process , the metal carbonyl nickel tetracarbonyl is used to produce pure nickel . Metal carbonyls serve as precursors for other complex compounds and for studying metal-metal bonds via the substitution of the carbon monoxide ligand, via oxidation and reduction reactions on the metal or via nucleophilic attack on the carbonyl carbon atom .
Metal carbonyls can be absorbed through skin contact, inhalation or ingestion. They are toxic and cause serious lung damage when inhaled. High exposure can lead to toxic pneumonia or toxic pulmonary edema . The decomposition product carbon monoxide, which binds to hemoglobin and thus impairs the transport of oxygen in the blood, is also toxic.
nomenclature
The nomenclature of the metal carbonyls depends on the charge of the complex, the number and type of central atoms and the number and type of ligands and their binding modes . Metal carbonyls occur in the form of neutral complexes, as positively charged metal carbonyl cations or as negatively charged metal carbonylate anions .
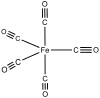
Mononuclear metal carbonyls contain only a single metal atom as a central atom. Apart from vanadium hexacarbonyl , only metals with an even atomic number such as chromium , iron , nickel and their homologues form neutral, mononuclear complexes. Polynuclear metal carbonyls are formed from metals with an odd atomic number and contain a metal-metal bond . Complexes with different central atoms but identical ligands in the same arrangement are called isoleptic .
The number of carbon monoxide ligands in a metal carbonyl complex is named with a Greek numeral followed by the word -carbonyl. Complexes that contain only carbon monoxide as a ligand are called pure or homoleptic metal carbonyls. If the complexes contain ligands other than carbon monoxide, the complexes are called mixed or heteroleptic metal carbonyls. Carbon monoxide has different binding modes in metal carbonyls. They differ in their hapticity and the bridging mode . The hapticity describes the number of carbon monoxide molecules that are directly bound to the central atom. It is named using the letter η n , which is placed in front of the name of the complex. The superscript n indicates the number of bound atoms. In the case of monohapto coordination, such as with terminally bound carbon monoxide, the hapticity is 1 and there is usually no separate designation. If carbon monoxide is bound to the metal via both the carbon and the oxygen atom, this is referred to as dihapto coordination η 2 .
The bridging mode describes the number of metal atoms that are bridged by a ligand atom. They are named with the letter μ m . The subscript m indicates the number of bridged metal atoms. In the case of two bridged centers, the subscript is sometimes not written out, so that μ corresponds to the notation μ 2 . In the complexes, carbon monoxide occurs terminally, bridging as μ 2 and facial as μ 3 and as a four-electron donor in a μ 3 as μ 2 -η 2 bridge. The tendency for the carbon monoxide ligand to form bridges decreases as the atomic radius of the central atom increases.
history
Early work
Justus von Liebig carried out the first attempts to convert carbon monoxide with metals in 1834. By passing carbon monoxide over molten potassium , he came up with a substance with the empirical formula KCO, which he called carbon oxide potassium . However, the compound shown is not a metal carbonyl, but the potassium salt of hexahydroxybenzene or the potassium salt of dihydroxyacetylene .
Paul Schützenberger succeeded in synthesizing the first real heteroleptic metal carbonyl complex in 1868. By passing chlorine and carbon monoxide over platinum black , he produced dicarbonyl dichloridoplatinum . Ludwig Mond , one of the co-founders of Imperial Chemical Industries , investigated in the years around 1890 together with Carl Langer and Friedrich Quincke various processes for the recovery of the chlorine lost in the Solvay process by means of nickel metals , oxides and salts. During the tests, they treated nickel with carbon monoxide, among other things. In doing so, they discovered that the resulting gas gave the gas flame of a Bunsen burner a greenish-yellow color and, when heated in the glass tube, formed a nickel mirror. The gas condensed to a colorless, water-clear liquid with a boiling point of 43 ° C. With this, the moon group had discovered nickel tetracarbonyl, the first pure homoleptic metal carbonyl complex. The high volatility of nickel tetracarbonyl, which is unusual for a metal compound, prompted Lord Kelvin to say that the moon had succeeded in "giving metals wings".
The following year, at the same time as Marcelin Berthelot , Mond reported on the preparation of iron pentacarbonyl using a related process. Mond recognized the economic potential of this class of compounds, which he used in the lunar process , and had his company continue to research related compounds. His staff Heinrich Hirtz and Matthewman Dalton Cowap put more metal carbonyls of cobalt , molybdenum and ruthenium and diiron nonacarbonyl . The structure elucidation of Dieisennonacarbonyls arising from iron pentacarbonyl by exposure to sunlight, only succeeded James Dewar and HO Jones 1906th year after the death of Mond in 1909, the chemistry of metal carbonyls was forgotten for a few years. In 1924, BASF began large-scale production of iron pentacarbonyl using a process developed by Alwin Mittasch and processed it into high-purity iron, so-called carbonyl iron , and iron oxide pigment .
Basic research by Walter Hieber
It was not until 1927 that A. Job and A. Cassal succeeded in synthesizing further homoleptic metal carbonyls with the preparation of chromium hexacarbonyl and tungsten hexacarbonyl . In the years that followed, from 1928 onwards, Walter Hieber's work made a decisive contribution to the further development of metal carbonyl chemistry. He examined them systematically and discovered, among other things, the Hieber base reaction , which led to the first known metal carbonyl hydrides and opened up synthetic routes to metal carbonyls such as dirhenium decacarbonyl . Hieber, who was director of the Inorganic Chemical Institute of the Technical University of Munich from 1934 , published 249 papers on metal carbonyl chemistry in four decades.
Technical processes according to Walter Reppe and Otto Roelen
In the 1930s, Walter Reppe , an industrial chemist and later board member of BASF , discovered a number of homogeneous catalytic processes, such as hydrocarboxylation to convert olefins or alkynes with carbon monoxide and water to produce products such as unsaturated carboxylic acids and their derivatives. For example, nickel tetracarbonyl or cobalt carbonyls are used as catalysts in these reactions . In addition, Reppe succeeded in the cyclotrimerization and tetramerization of acetylene and its derivatives to benzene and benzene derivatives as well as cyclooctatetraene using metal carbonyl catalysts . In the 1960s, BASF built a production facility for acrylic acid using the Reppe process. It was not until 1996 that more modern methods based on catalytic propylene oxidation replaced the process.
In 1938 Otto Roelen discovered the synthesis of fatty alcohols by means of homogeneous catalytic hydroformylation using cobalt carbonyls at what was then the Kaiser Wilhelm Institute for Coal Research . In this process, olefins are converted into aldehydes and alcohols on an industrial scale with carbon monoxide and hydrogen . By means of hydroformylation using modified metal carbonyls in the Ruhrchemie / Rhône-Poulenc process , several million tonnes of various products are produced every year.
Works by EO Fischer
In 1964, EO Fischer , who did his doctorate on metal carbonyl chemistry under Walter Hieber, discovered the first carbene complex by reacting tungsten hexacarbonyl with butyllithium , analogous to Hieber's base reaction . The Royal Swedish Academy of Sciences awarded his work the Nobel Prize in Chemistry in 1973 .
One subject of the organometallic research was substituted metal carbonyls in which carbon monoxide ligands were replaced by other ligands such as phosphines or cyclopentadienyl anions ( half-sandwich complexes ) . A mixed iridium-carbonyl complex ( Vaska's complex ) synthesized by Lauri Vaska shows a reversible binding of oxygen and serves as a model substance for the investigation of the oxygen transport in the blood. Rowland Pettit discovered in 1965 that metal carbonyls can serve to stabilize antiaromatic states. Metal carbonyls found increasing use in the field of organic chemistry. In 1971, the Pauson-Khand reaction was discovered, in which carbon monoxide reacts with an olefin and an alkyne to form substituted cyclopentenones . Dicobalt octacarbonyl served as the source of carbon monoxide . As early as 1973 it was possible to develop a catalytic variant of the process. In 1982, Wolfgang A. Herrmann succeeded in producing a mixed dinuclear metal carbonyl complex with a metal-metal double bond by oxidizing a carbon monoxide ligand with trimethylamine oxide .
Molecular Orbital Theory and Isolobal Analogy
The structure of metal carbonyls has long been the subject of speculation. For nickel tetracarbonyl, an ionic structure with a tetracarbonyl dianion [C 4 O 4 ] 2− was initially assumed. In 1921 , Irving Langmuir proposed a structure with a nickel-carbon double bond based on the principle of electroneutrality . Work by Linus Pauling in 1935 came to the conclusion that nickel tetracarbonyl is a tetrahedral complex with resonance structures with double bond character. Pauling gave the double bond character of the nickel-carbon bond due to the bond length of 182 pm as 78 percent. In 1981, Roald Hoffmann and Fukui Ken'ichi received the Nobel Prize in Chemistry for their concept of molecular orbital theory and the isolobal analogy , with which, among other things, the structures and chemistry of metal carbonyls can be elegantly described.
Modern research directions
The replacement of carbon monoxide with tailor-made ligands, such as the water-soluble tri- (sodium-meta-sulfonatophenyl) -phosphine , made it possible to produce mixed metal carbonyls. Their properties could be specifically adapted to the requirements of technical processes. Complexes of this type are used in industrial processes such as the Ruhrchemie / Rhône-Poulenc process for the industrial production of butanal from propene , hydrogen and carbon monoxide. In 2009, doctors investigated the use of metal carbonyls in cancer therapy , in which light-induced reduction is used to specifically release carbon monoxide in order to kill cancer cells in a controlled manner.
Occurrence

In the oxygen-rich terrestrial atmosphere , metal carbonyls are quickly subject to oxidation to metal oxide. It is discussed whether such complexes arose in the reducing hydrothermal environments of prebiotic prehistoric times and could have served as catalysts for the synthesis of critical biochemical compounds such as pyruvic acid . Various metal carbonyls such as iron, nickel and tungsten carbonyls have been detected as trace impurities in the gaseous vapors in the sewage sludge of municipal sewage treatment plants.
Carbon monoxide-containing complexes of hemoglobin and myoglobin are of biological importance . Hydrogenases partly contain iron-bound carbon monoxide.
Recent research identified iron pentacarbonyl as a possible driving force for volcanic eruptions and as part of the magma on terrestrial planets and the moon . In addition, iron pentacarbonyl is seen as a component of planetary carbon cycles.
When examining the infrared spectrum of the galactic center, astronomers detected carbon monoxide oscillations of iron carbonyls in interstellar dust clouds . Chinese researchers identified iron carbonyl clusters in Jiange H5 chondrites using infrared spectroscopy . They found four infrared stretching vibrations that they assigned to terminal and bridging carbon monoxide ligands.
Manufacturing
The substance class of metal carbonyls has been the subject of intensive organometallic research, especially since Hieber's work. Since then, chemists have developed many synthetic processes for the preparation of mononuclear metal carbonyls through to complex homo- and heterometallic clusters. Since some of these compounds are extremely sensitive to air, light, moisture and temperature, the use of the Schlenk technique is recommended for the production and handling of metal carbonyls . Furthermore, methods of representation under pressure of up to 500 bar in autoclaves lined with silver or copper have become established .
Direct conversion of metal with carbon monoxide
Nickel tetracarbonyl and iron pentacarbonyl can be produced by reacting finely divided metal with carbon monoxide according to the following equations:
Nickel tetracarbonyl forms with carbon monoxide already at 80 ° C and normal pressure , finely divided iron reacts at temperatures between 150 and 200 ° C and a carbon monoxide pressure of 50 to 200 bar. Carbonyls of other metals such as molybdenum hexacarbonyl , ruthenium pentacarbonyl or cobalt octacarbonyl can also be obtained in this way, but are usually produced using other synthetic routes.
Reduction of metal salts and oxides
Another way of presenting metal carbonyls is the reduction of metal halides with a reducing agent under carbon monoxide pressure. The reducing agents used include metallic copper or aluminum , hydrogen , carbon monoxide or metal alkyls such as triethylaluminum . Chromium hexacarbonyl is prepared in good yield from anhydrous chromium (III) chloride in benzene with aluminum as the reducing agent and aluminum chloride as the catalyst:
If carbon monoxide is used in excess under suitable conditions, it functions as a reducing agent. Walter Hieber and H. Fuchs succeeded in one of the first known syntheses using this process with the preparation of dirhenium decacarbonyl from the oxide in 1941.
When using metal oxides, carbon dioxide is formed as an oxidation product . The reduction of metal chlorides with carbon monoxide results in phosgene , for example in the preparation of osmium carbonyl chloride from the chloride. Carbon monoxide is suitable for the reduction of sulfides , which produces carbonyl sulfide .
When metal alkyls are used as the reducing agent, the alkyl radical is oxidatively coupled to form the dimer . Triethylaluminum or diethylzinc are suitable as metal alkyls ; the reaction takes place in diethyl ether as solvent.
The reduction of tungsten , molybdenum , manganese and rhodium salts is possible with lithium aluminum hydride as a reducing agent in diethyl ether.
The representation of vanadium hexacarbonyl achieved with sodium as a reducing agent in chelating solvents such as diglyme .
In the aqueous phase, for example, nickel or cobalt salts can be reduced with sodium dithionite . When exposed to carbon monoxide, the tetracarbonyl cobaltate (-I) (cobalt carbonylate) is quantitatively formed from cobalt salts.
Photo- and thermolysis
Photolysis or thermolysis of mononuclear carbonyls produces the carbonyls equipped with metal-metal bonds such as, for example, diiron nonacarbonyl (Fe 2 (CO) 9 ). Upon further heating, the complexes eventually break down into the metal and carbon monoxide.
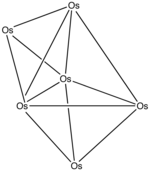
The thermal decomposition of triosmium dodecacarbonyl (Os 3 (CO) 12 ) yields higher nuclear osmium carbonyl clusters such as Os 4 (CO) 13 via Os 6 (CO) 18 to Os 8 (CO) 23 .
Oxidation of metal carbonyl anions
The dimer ditantalum dodecacarbonyl Ta 2 (CO) 12 can be obtained by the oxidation of a tantalum hexacarbonyl anion by means of a silver salt.
Salt metathesis
Through the salt metathesis , for example of KCo (CO) 4 with [Ru (CO) 3 Cl 2 ] 2 , mixed metallic metal carbonyls such as RuCo 2 (CO) 11 can be produced in a targeted manner.
Metal carbonyl cations and carbonylates
The preparation of ionic compounds is possible by oxidation or reduction from the neutral complexes. The cationic hexacarbonyl salts of manganese, technetium and rhenium can be prepared from the carbonyl halides under carbon monoxide pressure by reaction with a Lewis acid .
By using strong acids, gold carbonyl cations such as [Au (CO) 2 ] + , which are used as a catalyst for the carbonylation of olefins, were prepared . The cationic platinum carbonyl complex [Pt (CO) 4 ] + is accessible by working in so-called super acids such as antimony pentafluoride.
Metal carbonylates can be obtained, for example, by reducing binuclear complexes with sodium. A well-known example is the sodium salt of iron tetracarbonylate (Na 2 Fe (CO) 4 , Collman's reagent ), which is used in organic synthesis for the preparation of aldehydes by reacting with alkyl bromides or acid halides .
properties
Physical Properties
The mononuclear carbonyl complexes of the metals of the eighth subgroup are often colorless to yellowish liquids, which are highly volatile and highly toxic. The vapors of the nickel and iron carbonyls form explosive mixtures with air. The carbonyls of the metals of the sixth subgroup are colorless crystals that melt with decomposition. Vanadium hexacarbonyl, a 17-electron complex, is a blue-black solid.
The binuclear metal carbonyls of the first minor period are yellow to orange, those of the higher periods are colorless. Polynuclear metal carbonyls are yellow, red to black crystals; Triiron dodecacarbonyl (Fe 3 (CO) 12 ) forms deep green crystals. The crystalline metal carbonyls can be sublimed in vacuo , sometimes with decomposition.
Metal carbonyls are soluble in many organic solvents such as benzene , diethyl ether , acetone , glacial acetic acid and carbon tetrachloride , but not in water. In mineral acids , metal carbonyls dissolve with evolution of gas and the formation of metal salts.
Molecular Properties
binding
Metal carbonyls are particularly stabilized by the synergistic σ-donor / π-acceptor interactions of the carbon monoxide with the metal. As a result of these interactions, the electron density changes from the filled σ * orbital of the CO bond, the lone pair of electrons on the C atom, into the symmetry-equivalent, empty d orbitals of the metal and from filled metal orbitals into the empty π * orbitals of the CO multiple bond postponed.
As a result, the entire complex experiences an electron delocalization and thus an energetic stabilization through the nephelauxetic effect . There is also a π-donor interaction, which, however, is weak and therefore often neglected. According to Linus Pauling , the structure can be explained by the resonance of the following boundary structures.
Structures of small metal carbonyls
The structures of the metal carbonyls are primarily predicted by the VSEPR model and the 18-electron rule, for more complex structures by means of the isolobal concept , for whose development Roald Hoffmann received the Nobel Prize. Metal carbonyl fragments M (CO) n represent parts of octahedral building blocks which can be combined in analogy to the organic chemistry of the tetrahedral CH 3 , CH 2 or CH fragments. In the example of dimanganese decacarbonyl there are two d 7 Mn (CO) 5 fragments that are isolobal to the methyl radical CH 3 • in the sense of the isolobal analogy . Analogous to the combination of methyl radicals to ethane, these can be combined to form dimanganese decacarbonyl. The presence of isolobal-analogous fragments does not mean that the desired structures can be represented. In his Nobel Prize lecture, Hoffmann emphasized that the isolobal analogy is a useful but simple model and does not lead to success in certain cases.
Complexes with metals of higher periods tend to form fewer CO bridges than those of lower periods, which is due, among other things, to the greater splitting of the metal valence orbitals and the associated low-spin configuration according to ligand field theory .
Structure of metal carbonyl clusters
According to a definition by FA Cotton , clusters are molecules in which two or more metal atoms are not only bound to other non-metal atoms but also to themselves:
"Metal atom cluster compounds can be formally defined as those that contain a finite group of metal atoms and that are wholly, mainly, or at least in large part held together by direct bonds between the metal atoms, although some non-metal atoms may be closely associated with the cluster."
Depending on the size of the observed clusters, different approaches are used to describe the structures. Small clusters with two to six metal framework atoms follow the 18-electron rule (EAN, Effective Atomic Number Rule, Sidgwick Rule) with a few exceptions .
The structures of larger clusters with around five to twelve skeletal atoms can be predicted using modified Wade rules , the so-called Wade-Mingos rules (skeleton electron pair theory) . According to the isolobal rules, the complex fragments are converted into isolobal borohydride units .
"We will call two fragments isolobal if the number, the symmetry properties, the approximate energy and shape of the frontier orbitals and the number of electrons in them are similar - not identical, but similar."
Particularly large metal carbonyl clusters with more than twelve framework atoms already have a predominantly metallic character , so that they can be described more as metallic microcrystallites with chemisorbed carbon monoxide. The structure corresponds to a section from the metal grid .
Chemical properties
With a few exceptions, the metal carbonyls meet the 18-electron rule . The rule states that complexes with 18 valence electrons are stable, since the metals thus reach the electron number of the next higher noble gas . The rule can be applied to many complexes of transition metals. Metal carbonyls are important starting compounds for the preparation of other complexes. The most important reactions are the substitution of carbon monoxide by other ligands, the oxidation and reduction of the metal and reactions on carbon monoxide.
The substitution in 18-electron complexes occurs through a dissociative mechanism via a 16-electron stage. The substitution can be induced thermally or photochemically. In the photochemically induced substitution, an antibonding molecular orbital of the complex is occupied by light absorption, whereby the metal-carbon bond is weakened and the ligand is split off. In the case of metal carbonyl clusters whose metal-metal bonds can be cleaved by light, the substitution can be initiated by oxidizing a carbon monoxide ligand to carbon dioxide with an oxidizing agent such as trimethylamine oxide .
The carbon monoxide ligand can often easily be substituted by analogous ligands such as phosphines , cyanide ions (CN - ), olefins or the nitrosyl cation (NO + ). The reaction is advantageously carried out in solvents such as diethyl ether or acetonitrile , which can stabilize the intermediate stages formed as the oxygen or nitrogen donor ligand. Conjugated olefins can replace one carbon monoxide ligand in the complex for each olefinic double bond.
The dissociation energy is 105 kJ mol −1 for nickel tetracarbonyl and 155 kJ mol −1 for chromium hexacarbonyl.
The substitution in 17-electron complexes takes place according to an associative mechanism via a 19-electron stage.
The reaction rate according to the associative mechanism is in many cases higher than that of the dissociative mechanism. Vanadium hexacarbonyl reacts around a factor of 10 10 faster than tungsten hexacarbonyl. By means of electron-transfer catalysis , for example by catalytic oxidation of an 18 electron complex to 17 electron complex is possible to significantly increase the rate of substitution in the 18-electron complexes.
With reducing agents such as metallic sodium in ammonia , the metal carbonyls form metal carbonylate anions. In the case of multinuclear complexes, the metal-metal bond can split .
In the Hieber base reaction, the hydroxide ion reacts with metal carbonyls such as iron pentacarbonyl by nucleophilic attack on the carbonyl carbon atom, releasing carbon dioxide and forming metal carbonylates.
In a further step, iron carbonyl hydride, a very temperature and air-sensitive complex hydride, can be synthesized, which Walter Hieber discovered in 1931:
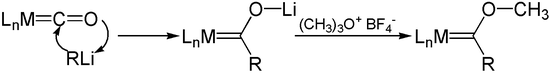
Metal carbonyl hydrides such as cobalt carbonyl hydride (HCo (CO) 4 ) are accessible through the Hieber base reaction . Organolithium compounds react with metal carbonyls analogously to Hieber's base reaction . By subsequent alkylation with Meerwein salt trimethyloxonium be Fischer carbenes received.
Metal carbonyls react with halogens with oxidative halogenation . In the case of multinuclear complexes, oxidative cleavage of metal-metal bonds can occur.
Unsaturated compounds or mercury can insert into the metal-metal bond of polynuclear metal carbonyls.
Sodium tetracarbonyl ferrate , known as Collman's reagent , is used in organic synthesis to prepare aldehydes from alkyl bromides . In the first step, the anion reacts with alkyl bromides to form sodium bromide and the alkyl complex.
The aldehyde is released by adding triphenylphosphine and then acidifying it with acetic acid .
The alkyl complex formed primarily can be converted into the acyl complex by triphenylphosphine and into the carboxylic acid by oxidation with oxygen and subsequent acidification . The acyl complex is converted into the carboxylic acid chlorides by oxidation with halogens .
Lewis acids such as trialkylaluminum complexes can bind to the oxygen of the carbon monoxide ligand. In this case, the oxygen acts as a Lewis base.
use
In technical processes in which gases containing carbon monoxide are used under pressure, the formation of metal carbonyls can regularly be observed if the material is improperly selected, combined with corrosion due to the formation of iron and nickel carbonyls. In the past, when using hard coal gas containing carbon monoxide, a red-brown, iron-containing coating was observed on incandescent mantles , which originated from the oxidation of iron carbonyl contained in the gas and which reduced the luminosity . In heterogeneous catalytic processes, deactivation of the contact as a result of irreversible decomposition of metal carbonyls on the contact surface is observed. For the storage of test gases containing carbon monoxide , we recommend the use of aluminum bottles to avoid the formation of iron pentacarbonyl.
Moon procedure
The moon procedure consists of three steps. In the first step, nickel oxide is reacted with synthesis gas at 200 ° C in order to reduce the oxide to metal. The impure nickel is reacted with excess carbon monoxide in the cooler zone of a transport apparatus at 50-60 ° C. to form nickel tetracarbonyl. Impurities such as iron and cobalt remain as solids. The mixture of excess carbon monoxide and nickel tetracarbonyl is then heated to 220-250 ° C., during which the nickel tetracarbonyl decomposes to metallic nickel and carbon monoxide. In 1910, the Mond Nickel Company Ltd. already produced 3000 tons of nickel with a purity of 99.9 percent. The process is used to this day, with around 30,000 tons per year being produced in North America alone.
The decomposition can be controlled in a targeted manner in order to either produce nickel powder or to coat an existing substrate with nickel. In addition to the production of pure nickel, this process is also suitable for coating other metals with nickel.
Carbonyl iron
According to a similar process, iron pentacarbonyl is used to manufacture carbonyl iron , a high-purity metal powder that is used, among other things, for the manufacture of coil cores , diamond tools , pigments , as a dietary supplement and in the manufacture of radar- absorbing materials in stealth technology and metal injection molding .
For the production of soft magnetic coil cores, the carbonyl iron particles are usually bound in an insulating plastic matrix. This reduces the eddy currents, especially at high frequencies.
Hydroformylation

(1) Elimination of a CO with formation of a 16-electron species.
(2) Uptake of the olefin in free coordination sites.
(3) Formation of the 16-electron alkyl complex by rearrangement.
(4) Admission of CO to vacant coordination positions.
(5) Insertion into the metal-carbon bond of the alkyl radical to form a 16-electron acyl complex.
(6) Oxidative addition of hydrogen.
(6) Release of the aldehyde, restoration of the active species.
(7) The catalytic cycle closes with the formation of the starting complex.
(8) As a side reaction, the 16-electron complex can take up one molecule of carbon monoxide in an equilibrium reaction.
In 1938 Otto Roelen discovered hydroformylation with cobalt carbonyl hydride as the active catalyst species. It is used for the large-scale synthesis of fatty alcohols that are used for detergent production. Today several million tons of Oxo products are obtained through this process. The hydroformylation is one of the atom-economically favorable processes, especially if the reaction proceeds with high regioselectivity . The more active mixed rhodium carbonyl hydrides displaced the cobalt carbonyl hydrides originally used in the oxo process.
Reppe chemistry
Reppe chemistry is understood to mean working with acetylene under elevated pressure, metal carbonyls and hydrogen carbonyls being used as catalysts. An important reaction is hydrocarboxylation , using the example of acrylic acid and acrylic acid ester production:
Cyclization and cyclizing polymerization using the example of benzene and cyclooctatetraene production uses metal carbonyls as catalysts:
By cotrimerization of hydrocyanic acid with acetylene can be pyridine and its derivatives produced. Reppe chemistry gives rise to numerous intermediate products in industrial chemistry, which are used as starting products for paints , adhesives , injection molding compounds , foams , textile fibers or medicines in further process steps.
Molecules that release carbon monoxide
Carbon monoxide has a biological effect as a messenger substance that humans produce in quantities of around 3–6 cm 3 per day. Inflammation and conditions that affect red blood cell hemolysis can increase the amount significantly. Carbon monoxide has anti-inflammatory activity in the cardiovascular system and protects the tissue from reduced blood flow and apoptosis . Carbon monoxide releasing molecules are chemical compounds that release carbon monoxide in a cellular environment to act as a therapeutic agent. Modified metal carbonyl complexes such as acyloxybutadiene iron carbonyl complexes or the ruthenium (II) complex Ru (gly) Cl (CO) 3 release carbon monoxide in the organism and show similar biological effects as the carbon monoxide itself.
Other uses
Iron pentacarbonyl catalyzes the water gas shift reaction
according to the following mechanism:
The process has no technical significance.
Walter Ostwald , the son of Wilhelm Ostwald , used iron pentacarbonyl as an anti-knock agent in gasoline, the so-called Motalin . However, due to the build-up of iron oxides in the engine compartment and on the spark plugs and the problems associated with them, the refineries discontinued this use.
When producing pure hydrogen from synthesis gas , the formation of copper carbonyls is used in the last stage of carbon monoxide separation. For this purpose, the pre-cleaned synthesis gas is passed through an aqueous solution containing copper chloride, in which carbon monoxide binds to form a copper carbonyl ([(H 2 O) 2 ClCuCO]).
The thermal decomposition of metal carbonyl clusters on carrier materials is used to produce contacts with a defined metal particle size, which are used in heterogeneous catalysis . In addition to the deposition on catalytically active surfaces, the conversion of mononuclear complexes into metal carbonyl clusters in the cavities of zeolites is investigated.
Related links
Many ligands isoelectronic with carbon monoxide form homoleptic analogs to the metal carbonyls or heteroleptic carbonyl complexes. Triphenylphosphine ligands can be varied within wide limits and their properties can be tailored for different applications by substitution.
Phosphane complexes
In all metal carbonyls, the carbon monoxide ligands can be wholly or partly substituted by organophosphorus ligands of the PR 3 type . For example, the Fe (CO) 5-x (PR 3 ) x series is known for up to three phosphine ligands. Phosphorus trifluoride (PF 3 ) behaves similarly and forms homoleptic analogues of the metal carbonyls, for example the volatile stable complexes Fe (PF 3 ) 5 and Co 2 (PF 3 ) 8 .
Nitrosyl complexes
Nitrosyl compounds with nitric oxide (NO) as a ligand are numerous, although homoleptic derivatives are not known. In relation to carbon monoxide, nitrogen monoxide is a stronger acceptor. Well-known nitrosyl-carbonyl complexes are CoNO (CO) 3 and Fe (NO) 2 (CO) 2 .
Thiocarbonyl complexes
Complexes with carbon monosulfide (CS) are known but rare. Part of the reason why such complexes are difficult to make is due to the fact that carbon monosulfide is unstable. The synthesis of thiocarbonyl complexes therefore requires special synthetic routes, such as the reaction of sodium tetracarbonyl ferrate with thiophosgene :
The analogous complexes of carbon monoselenide and carbon monotelluride are very rare.
Isonitrile complexes
Isonitriles form extensive families of complexes related to the metal carbonyls. Typical isonitrile ligands are methyl isocyanide and tert-butyl isocyanide. A special case is trifluoromethyl isocyanide (CF 3 NC), an unstable molecule that forms stable complexes whose behavior is similar to that of metal carbonyls.
toxicology
The toxicity of a metal carbonyl is due to the toxicity of the carbon monoxide and the metal and the volatility and instability of the complex. Exposure occurs through inhalation, in the case of liquid metal carbonyls through ingestion or, due to the good fat solubility, through skin absorption . Most of the toxicological experience comes from poisoning with nickel tetracarbonyl and iron pentacarbonyl. Nickel tetracarbonyl is one of the strongest inhalation poisons.
Inhalation of nickel tetracarbonyl causes acutely non-specific symptoms similar to carbon monoxide poisoning such as nausea , dry cough , headache , fever and dizziness . After a while, severe pulmonary symptoms such as cough, tachycardia , cyanosis , or digestive tract problems develop . In addition to pathological changes in the lungs, such as those caused by metalation of the alveoli and the brain, damage to the liver, kidneys, adrenal glands and spleen are observed. Metal carbonyl poisoning often results in long-lasting convalescence .
Chronic exposure through inhalation of low concentrations of nickel tetracarbonyl can cause neurological symptoms such as insomnia, headache, dizziness and memory loss. Nickel tetracarbonyl is considered to be carcinogenic , with a period of 20 to 30 years between the onset of exposure and the clinical manifestation of the cancer .
Analytical characterization

Important analytical techniques for the investigation and characterization of metal carbonyls are infrared spectroscopy and 13 C-NMR spectroscopy . The information that these two techniques provide moves on different time scales. Infrared-active molecular vibrations such as CO stretching vibrations are often fast compared to intramolecular processes, while NMR transitions are slower and take place in the time domain of intra- or intermolecular ligand exchange processes. This can result in NMR data providing time-averaged information.
For example, the investigation of dicobalt octacarbonyl (Co 2 (CO) 8 ) by means of infrared spectroscopy yields bands for 13 different CO oscillations and thus considerably more than is to be expected for 8 substituents. The reason is the simultaneous presence of different isomers, for example with and without bridging CO ligands. In contrast, the 13 C-NMR investigation of the same substance only yields a single signal with a chemical shift of 204 ppm. This indicates that the isomers are rapidly converting into one another.
Infrared spectroscopy
The investigation of metal carbonyls by means of infrared spectroscopy provides a wide range of information, for example about the binding modes of carbon monoxide, the complex geometry and the charge of the complex. In the case of heteroleptic metal carbonyls, infrared spectroscopy also provides information about the properties and bonding conditions of the ligand trans to the carbon monoxide .
The wave number of the CO stretching vibration ν CO of the free carbon monoxide is 2143 cm −1 , the number and type of CO stretching vibration of the metal carbonyls allow conclusions to be drawn about the structure of the complex. In the case of metal carbonylates, for example, the formal negative charge on the metal causes increased backbinding into the anti-binding orbitals of the carbon monoxide ligand and thus a weakening of the carbon-oxygen bond, which in such complexes has the effect of shifting the infrared absorption to lower wavenumbers. Cationic complexes shift these accordingly to higher wavenumbers. Apart from the CO stretching vibrations, no further vibrations are to be expected in the observed wave number range from approx. 1800 to 2000 cm −1 . Terminal and bridging ligands in the complex can be distinguished using infrared spectroscopy.
| component | ν CO (cm −1 ) |
|---|---|
| CO | 2143 |
| Ti (CO) 6 2− | 1748 |
| V (CO) 6 - | 1859 |
| Cr (CO) 6 | 2000 |
| Mn (CO) 6 + | 2100 |
| Fe (CO) 6 2+ | 2204 |
| Fe (CO) 5 | 2022, 2000 |
In addition to the frequency, the number of vibration bands allows conclusions to be drawn about the spatial structure of the complex. The number of vibrational modes of a metal carbonyl complex can be determined using group theory . Only oscillation modes that are accompanied by a change in the dipole moment are infrared active and can be observed. The number of observable IR transitions, but not their energies, can thus be predicted.
Octahedral complexes such as Cr (CO) 6 show only a single ν CO band in the infrared spectrum due to the chemical consistency of all ligands . Spectra of complexes with lower symmetry, on the other hand, are more complex. The infrared spectrum of the diiron nonacarbonate Fe 2 (CO) 9 , for example, shows CO bands at 2082, 2019 and 1829 cm −1 .
In the case of multinuclear complexes, the position of the ν CO oscillation allows conclusions to be drawn about the coordination geometry of the carbon monoxide. For bridging μ 2 -coordinating ligands, the position of the ν CO oscillation is shifted by about 100 to 200 cm −1 to lower wavenumbers compared to the ν CO oscillation of carbon monoxide in the terminal position. This effect is even more pronounced for μ 3 -bridging carbon monoxide.
Typical values for rhodium clusters are:
| Metal carbonyl | ν CO , µ 1 (cm −1 ) | ν CO , µ 2 (cm −1 ) | ν CO , µ 3 (cm −1 ) |
|---|---|---|---|
| Rh 2 (CO) 8 | 2060, 2084 | 1846, 1862 | |
| Rh 4 (CO) 12 | 2044, 2070, 2074 | 1886 | |
| Rh 6 (CO) 16 | 2045, 2075 | 1819 |
Nuclear magnetic resonance spectroscopy
A useful method for investigating metal carbonyls is nuclear magnetic resonance spectroscopy of carbon nuclei such as 13 C, oxygen nuclei such as 17 O or metal nuclei such as 195 Pt, in mixed carbonyl complexes of phosphorus nuclei 31 P and hydrogen nuclei 1 H. To improve the resolution, 13 C-NMR- Spectroscopy complexes with artificially enriched 13 C-carbon monoxide ligands are used. Typical values of the chemical shift are 150 to 220 ppm for terminally bound ligands and 230 to 280 ppm for bridging ligands. The shielding increases with the atomic number of the central atom.
Nuclear magnetic resonance spectroscopy is suitable for the experimental determination of complex dynamics phenomena . The activation energy can be determined from the measurement of the temperature dependence of changes in ligand positions. Iron pentacarbonyl, for example, only gives a single 13 C-NMR signal. The reason for this is the rapid change of ligand position due to Berry pseudorotation .
Mass spectrometry
The investigation of metal carbonyls using mass spectrometry allows conclusions to be drawn about the structure of the complexes. The most commonly used ionization technique is electrospray ionization (ESI). Solutions of charged substances are sprayed, ionized and the droplets are dried so that ions of the analyte remain. This method is particularly suitable for ionic metal carbonyls. Neutral metal carbonyls can be converted into ionic species by means of derivatization , for example by reaction with alcoholates . The reaction of the metal carbonyls with azides produces ionic isocyanate derivatives with the release of nitrogen . Metal carbonyls give easily interpretable spectra, since the dominant fragmentation process is the loss of the carbonyl ligands.
In electrospray ionization mass spectrometry, the fragmentation of the complexes can be varied by adjusting the voltage. In this way, the molar mass of the original complex can be determined as well as information about the structural rearrangements of cluster cores that partially or completely lose their carbon monoxide ligands under thermolytic conditions.
Other methods such as matrix-assisted laser desorption / ionization (MALDI), which have proven themselves for the mass spectrometry of high molecular weight substances, provide little useful information for the mass spectrometric investigation of metal carbonyls. The investigated compounds, such as the iridium carbonyl cluster Ir 4 (CO) 12 , formed clusters with up to 24 iridium and 30 to 35 carbonyl ligands under the conditions of the investigation, whereby the molecular ion peak was not detected.
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1780-1822.
- N. Krause : Organometallic chemistry - Selective syntheses with organometallic compounds. Spectrum Academic Publishing House, ISBN 3-86025-146-5 .
- Christoph Elschenbroich : Organometallic chemistry. 6th edition. BG Teubner Verlag, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , pp. 330-352.
- PJ Dyson, JS McIndoe: Transition Metal Carbonyl Cluster Chemistry. Routledge Chapman & Hall, 2000, ISBN 90-5699-289-9 .
- D. Astruc : Organometallic Chemistry and Catalysis. Verlag Springer, Berlin / Heidelberg 2007, ISBN 978-3-540-46128-9 .
- WA Herrmann : 100 years of metal carbonyls. A chance discovery makes history , in: Chemistry in our time , Volume 22, 1988, pp 113-122; doi : 10.1002 / ciuz.19880220402
Web links
Individual evidence
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1780.
- ^ F. Albert Cotton: Proposed nomenclature for olefin-metal and other organometallic complexes. In: Journal of the American Chemical Society. 90, 1968, pp. 6230-6232 ( doi : 10.1021 / ja01024a059 ).
- ^ A. Salzer: Nomenclature of Organometallic Compounds of the Transition Elements. In: Pure and Applied Chemistry. 71, 1999, pp. 1557-1585 ( doi : 10.1351 / pac199971081557 ).
- ^ WE Trout: The metal carbonyls. In: J. Chem. Educ. 1937, 14 (10), p. 453 ( doi : 10.1021 / ed014p453 ).
- ↑ a b c d e f g W. A. Herrmann : 100 years of metal carbonyls. A chance discovery makes history. In: Chemistry in Our Time . 1988, 22nd year, No. 4, pp. 113-122 ( doi : 10.1002 / ciuz.19880220402 ).
- ↑ P. Schützenberger in: Ann. Chim. Phys. (Paris) 1868, 15, pp. 100-106.
- ↑ L. Mond, C. Langer, F. Quincke: Action of Carbony Monoxide on Nickel. In: J. Chem. Soc. 1890, 57, pp. 749-753 ( doi : 10.1039 / CT8905700749 ).
- ^ W. Gratzer: Metal takes wing. In: Eurekas and Euphorias: The Oxford Book of Scientific Anecdotes. Oxford Univ. Press, 2002, ISBN 0-19-280403-0 , p. 265.
- ^ Ludwig Mond, Heinrich Hirtz, Matthewman Dalton Cowap: Note on a Volatile Compound of Cobalt With Carbon Monoxide. In: Chem. News. Vol. 98, 1908, p. 165; Chem. Abs. Vol. 2, 1908, p. 3315.
- ↑ James Dewar, HO Jones: The Physical and Chemical Properties of Iron Carbonyl. In: Proc. R. Soc. Lond. A . December 6, 1905, 76, pp. 558-577 ( doi : 10.1098 / rspa.1905.0063 ).
- ↑ a b c d e Alwin Mittasch: About iron carbonyl and carbonyl iron. In: Angewandte Chemie . Volume 41, Issue 30, 1928, pp. 827-833 ( doi : 10.1002 / anie.19280413002 ).
- ^ F. Basolo: From Coello to inorganic chemistry: a lifetime of reactions. P. 101 ( limited preview in Google Book search).
- ^ RA Sheldon: Chemicals from synthesis gas: catalytic reactions of CO and H 2 . Volume 2, p. 106 ( limited preview in Google Book search).
- ↑ Dirk Steinborn : Fundamentals of organometallic complex catalysis. Teubner, Wiesbaden 2007, ISBN 978-3-8351-0088-6 , p. 83. ( limited preview in the Google book search).
- ^ L. Watts, R. Pettit: Chemistry of Cyclobutadiene-iron Tricarbonyl. Werner Centennial, Chapter 34, 1967, pp. 549-554; Advances in Chemistry. Volume 62, January 1, 1967 ( doi : 10.1021 / ba-1967-0062.ch034 ).
- ^ Pauson-Khand-type reaction mediated by Rh (I) catalysts. (PDF file; 202 kB).
- ↑ WA Herrmann, J. Plank, Ch. Bauer, ML Ziegler, E. Guggolz, R. Alt: Metallcarbonyl-Synthesen. XI. Transition metal – methylene complexes. XXVI. On the reactivity of the half-sandwich complex [η 5 −C 5 Me 5 ) Rh (CO) 2 towards Bronsted acids, halogens and trimethylamine oxide. In: Journal of Inorganic and General Chemistry . Volume 487, Issue 1, April 1982, pp. 85-110 ( doi : 10.1002 / zaac.19824870109 ).
- ^ I. Langmuir : Types of Valence. In: Science . 1921, 54, pp. 59-67 ( doi : 10.1126 / science.54.1386.59 ).
- ↑ L. Pauling: The nature of the chemical bond. 3. Edition. Verlag Chemie, Weinheim 1968, ISBN 3-527-25217-7 .
- ^ A b c R. Hoffmann: Building Bridges between Inorganic and Organic Chemistry. (PDF file; 307 kB), Nobel lecture, December 8, 1981.
- ↑ Hendrik Pfeiffer, Alfonso Rojas, Johanna Niesel, Ulrich Schatzschneider: Sonogashira and "Click" reactions in the N-terminal and side chain functionalization of peptides with [Mn (CO) 3 (tpm)] + -based CO releasing molecules tpm = tris (pyrazolyl) methanes. In: Dalton Trans. , 2009, pp. 4292-4298 ( doi : 10.1039 / b819091g ).
- ↑ Gilles Gasser, Oliver Brosch, Alexandra Ewers, Thomas Weyhermüller, Nils Metzler-Nolte: Synthesis and characterization of hetero-bimetallic organometallic phenylalanine and PNA monomer derivatives. In: Dalton Trans. , 2009, pp. 4310-4317 ( doi : 10.1039 / b819169g ).
- ↑ George D. Cody, Nabil Z. Boctor, Timothy R. Filley, Robert M. Hazen, James H. Scott, Anurag Sharma, Hatten S. Yoder Jr .: Primordial Carbonylated Iron-Sulfur Compounds and the Synthesis of Pyruvate. In: Science . August 25, 2000, Vol. 289, No. 5483, pp. 1337-1340 ( doi : 10.1126 / science.289.5483.1337 ).
- ↑ J. Feldmann: Determination of Ni (CO) 4 , Fe (CO) 5 , Mo (CO) 6 , and W (CO) 6 in sewage gas by using cryotrapping gas chromatography inductively coupled plasma mass spectrometry. In: J Environ Monit . 1999, 1: pp. 33-37 ( doi : 10.1039 / A807277I ).
- ↑ JN Moore, PA Hansen, RM Hochstrasser: Iron-carbonyl bond geometries of carboxymyoglobin and carboxyhemoglobin in solution determined by picosecond time-resolved infrared spectroscopy. In: Proc. Natl. Acad. Sci. USA . July 1988, Vol. 85, pp. 5062-5066 ( PMC 281688 (free full text)).
- ↑ C. Tard, CJ Pickett: Structural and Functional Analogues of the Active Sites of the [Fe], [NiFe], and [FeFe] hydrogenases. In: Chem. Rev. 2009, 109, pp. 2245-2274 ( doi : 10.1021 / cr800542q ).
- ↑ MM Hirschmann: Fe-carbonyl is a key player in planetary magmas. In: Proceedings of the National Academy of Sciences. 110, 2013, pp. 7967-7968 ( doi : 10.1073 / pnas.1305237110 ).
- ↑ AGGM Tielens, DH Wooden, LJ Allamandola, J. Bregman, FC Witte Born: The infrared spectrum of the Galactic center and the composition of interstellar dust. In: Astrophys J. April 10, 1996, 461 (1 Pt 1), pp. 210-222 ( PMID 11539170 ).
- ^ Y. Xu, X. Xiao, S. Sun, Z. Ouyang: IR Spectroscopic Evidence of Metal Carbonyl Clusters in the Jiange H5 Chondrite. In: Lunar and Planetary Science . 1996, 27, pp. 1457-1458 ( PDF file; 471 kB ).
- ↑ a b c d metal carbonyls. In: Huheey, E. Keiter, R. Keiter: Inorganic Chemistry. 2nd Edition. de Gruyter, Berlin / New York 1995.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1790.
- ^ W. Hieber, H. Fuchs in: Z. anorg. allg. Chem. 1941, 248, p. 256.
- ^ A b c d A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1780-1822.
- ↑ Unkrig, W .; Schmitt, M .; Kratzert, D .; Himmel, D .; Krossing, I .: Synthesis and characterization of crystalline niobium and tantalum carbonyl complexes at room temperature in Nature Chemistry 12 (2020) 647-653, doi : 10.1038 / s41557-020-0487-3 .
- ^ E. Roland and H. Vahrenkamp: Two new metal carbonyls: Representation and structure of RuCo 2 (CO) 11 and Ru 2 Co 2 (CO) 13 . In: Chemical Reports . March 1985, Volume 118, Issue 3, pp. 1133-1142 ( doi : 10.1002 / cber.19851180330 ).
- ↑ Q. Xu, Y. Imamura, M. Fujiwara, Y. Souma: A New Gold Catalyst: Formation of Gold (I) Carbonyl, [Au (CO) n ] + (n = 1, 2), in Sulfuric Acid and Its Application to Carbonylation of Olefins. In: J. Org. Chem. 1997, 62 (6), pp. 1594-1598 ( doi : 10.1021 / jo9620122 ).
- ↑ a b c d e Peter Paetzold : Chemistry: An introduction. de Gruyter, 2009, ISBN 978-3-11-020268-7 , p. 870 ( limited preview in the Google book search).
- ^ Structure from The Cambridge Crystallographic Data Center (CCDC) .
- ↑ FA Cotton: Transition-metal compounds containing clusters of metal atoms. In: Q. Rev. Chem. Soc. 1966, 20, pp. 389-401 ( doi : 10.1039 / QR9662000389 ).
- ↑ Original English text: Metal atom cluster compounds can be formally defined as those containing a finite group of metal atoms which are held together entirely, mainly or at least to a significant extent, by bonds directly between the metal atoms even though some non-metal atoms may be associated intimately with the cluster .
- ↑ Original English text: We will call two fragments isolobal if the number, symmetry properties, approximate energy and shape of the frontier orbitals and the number of electrons in them are similar - not identical, but similar .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1781.
- ^ HH Ohst, JK Kochi: Electron-transfer catalysis of ligand substitution in triiron clusters. In: J. Am. Chem. Soc. 1986, 108 (11), pp. 2897-2908 ( doi : 10.1021 / ja00271a019 ).
- ↑ James M. Burlitch, Robert B. Petersen: Variable site of Lewis basicity of π-C 5 H 5 W (CO) −3 in complexes with (C 6 H 5 ) 3 In and (C 6 H 5 ) 3 Al. In: Journal of Organometallic Chemistry. 24, 1970, pp. C65-C67 ( doi : 10.1016 / S0022-328X (00) 84476-9 ).
- ↑ H. Schön: Handbuch der Reinsten Gase. 1st edition. Verlag Springer, Berlin / Heidelberg 2005, ISBN 3-540-23215-X .
- ↑ Dmitri S. Terekhov, Nanthakumar Victor Emmanuel: Direct extraction of nickel and iron from laterite ores using the carbonyl process. In: Minerals Engineering. 54, 2013, pp. 124–130 ( doi : 10.1016 / j.mineng.2013.07.008 ).
- ↑ Tom Jennemann: Nickel powder buyers in crisis as supply dries up. In: American Metal Market. May 21, 2010, accessed June 21, 2014 .
- ^ SJ Fairweather-Tait, B. Teucher: Iron and Calcium Bioavailability of Fortified Foods and Dietary Supplements. In: Nutrition Reviews . 2001, 60, pp. 360-367 ( doi : 10.1301 / 00296640260385801 ).
- ↑ D. Richardson: Stealth Fighter Planes: Deceiving and Camouflaging in the Air. Verlag Dietikon, Zurich 2002, ISBN 3-7276-7096-7 .
- ↑ Madina A. Abshinova, Alexander V. Lopatin, Natalia E. Kazantseva, Jarmila Vilčáková, Petr Sáha: Correlation between the microstructure and the electromagnetic properties of carbonyl iron filled polymer composites. In: Composites Part A: Applied Science and Manufacturing. 38, 2007, pp. 2471-2485 ( doi : 10.1016 / j.compositesa.2007.08.002 ).
- ↑ Ernst Wiebus, Boy Cornils: The large-scale oxo synthesis with immobilized catalyst. In: Chemical Engineer Technology. 66, 1994, pp. 916-923 ( doi : 10.1002 / cite.330660704 ).
- ↑ A. Gossauer: Structure and reactivity of biomolecules. Helvetica Chimica Acta, Zurich 2006, ISBN 3-906390-29-2 , p. 155.
- ^ G. Wilke : Organo Transition Metal Compounds as Intermediates in Homogenous Catalytic Reactions. (PDF file; 381 kB).
- ↑ Tony R. Johnson, Brian E. Mann, James E. Clark, Roberta Foresti, Colin J. Green, Roberto Motterlini: Carbonyl Metal Complexes - A New Class of Pharmaceuticals? In: Angewandte Chemie. 115, 2003, pp. 3850-3858 ( doi : 10.1002 / anie.200301634 ).
- ↑ Steffen Romanski, Birgit Kraus, Ulrich Schatzschneider, Jörg-Martin Neudörfl, Sabine Amslinger, Hans-Günther Schmalz: Acyloxybutadiene-Fe (CO) complexes as enzymatically activated, CO-releasing molecules (ET-CORMs). In: Angewandte Chemie. 123, 2011, pp. 2440-2444 ( doi : 10.1002 / anie.201006598 ).
- ↑ P. Sawle: Bioactive Properties of Iron-Containing Carbon Monoxide-releasing Molecules. In: Journal of Pharmacology and Experimental Therapeutics. 318, 2006, pp. 403-410 ( doi : 10.1124 / jpet.106.101758 ).
- ^ Allen D. King, RB King, DB Yang: Homogeneous catalysis of the water gas shift reaction using iron pentacarbonyl. In: Journal of the American Chemical Society. 102, 1980, pp. 1028-1032 ( doi : 10.1021 / ja00523a020 ).
- ^ Richard M. Laine, Robert G. Rinker, Peter C. Ford: Homogeneous catalysis by ruthenium carbonyl in alkaline solution: the water gas shift reaction. In: Journal of the American Chemical Society. 99, 1977, pp. 252-253, doi : 10.1021 / ja00443a049 .
- ^ RH Crabtree : The Organometallic Chemistry of the Transition Metals. Publisher John Wiley & Sons, 2009, ISBN 978-0-470-25762-3 .
- ^ H. Offermanns : The other Ostwald. In: News from chemistry . 2009 , 57, pp. 1201-1202 ( doi : 10.1002 / nadc.200970167 ).
- ↑ Jonathan Phillips, James A. Dumestic: Production of supported metal catalysts by the decomposition of metal carbonyls (review). In: Applied Catalysis. 9, 1984, pp. 1-30 ( doi : 10.1016 / 0166-9834 (84) 80034-2 ).
- ^ G. Schmid : Clusters and Colloids. From theory to applications. Verlag Wiley-VCH, 1994, ISBN 3-527-29043-5 .
- ^ A b Robert H. Crabtree : The Organometallic Chemistry of the Transition Metals. Publisher John Wiley & Sons, 2009, ISBN 978-0-470-25762-3 .
- ^ RB King, A. Efraty: Metal complexes of fluorophosphines. II. Use of tetrakis (trifluorophosphine) nickel as a source of trifluorophosphine in the synthesis of metal-trifluorophosphine complexes. In: Journal of the American Chemical Society. 94, 1972, pp. 3768-3773 ( doi : 10.1021 / ja00766a017 ).
- ↑ Trevor W. Hayton, Peter Legzdins, W. Brett Sharp: Coordination and Organometallic Chemistry of Metal-NO Complexes. In: Chemical Reviews. 102, 2002, pp. 935-992 ( doi : 10.1021 / cr000074t ).
- ^ A b W. Petz: 40 years of transition-metal thiocarbonyl chemistry and the related CSe and CTe compounds. In: Coordination Chemistry Reviews . 252, 2008, pp. 1689-1733 ( doi : 10.1016 / j.ccr.2007.12.011 ).
- ^ Richard S. Brief, Robert S. Ajemian, Robert G. Confer: Iron Pentacarbonyl: Its Toxicity, Detection, and Potential for Formation. In: American Industrial Hygiene Association Journal. 28, 1967, pp. 21-30 ( doi : 10.1080 / 00028896709342481 ).
- ↑ B. Madea: Forensic Medicine. Findings - reconstruction - assessment. Springer-Verlag, 2003, ISBN 3-540-43885-8 .
- ↑ a b J. M. Stellman: Encyclopaedia of Occupational Health and Safety. International Labor Org, 1998, ISBN 91-630-5495-7 .
- ↑ G. Mehrtens, M. Reichenbach, D. Höffler, Günter G. Mollowitz: The accident man: Assessment of the consequences of accidents at work, private accidents and occupational diseases. Verlag Springer, Berlin / Heidelberg 1998, ISBN 3-540-63538-6 .
- ^ FA Cotton: Chemical Applications of Group Theory . 3rd edition. Wiley Interscience, 1990, ISBN 0-471-51094-7 .
- ^ RL Carter: Molecular Symmetry and Group Theory . Wiley, 1997, ISBN 0-471-14955-1 .
- ^ DC Harris, MD Bertolucci: Symmetry and Spectroscopy: Introduction to Vibrational and Electronic Spectroscopy . Oxford University Press, 1980, ISBN 0-19-855152-5 .
- ↑ AD Allian, Y. Wang, M. Saeys GM Kuramshina, M. Garland: The combination of deconvolution and density functional theory for the mid-infrared vibrational spectra of stable and unstable rhodium carbonyl clusters . In: Vibrational Spectroscopy . tape 41 , 2006, p. 101–111 , doi : 10.1016 / j.vibspec.2006.01.013 .
- ^ R. Bramley, BN Figgis, RS Nyholm: 13C and 170 nmr spectra of metal carbonyl compounds. In: Transactions of the Faraday Society. 58, 1962, p. 1893 ( doi : 10.1039 / TF9625801893 ).
- ↑ Christoph Elschenbroich : Organometallchemie. 6th edition. BG Teubner Verlag, Wiesbaden 2008, ISBN 978-3-8351-0167-8 .
- ^ E. Riedel, R. Alsfasser, C. Janiak, TM Klapötke: Modern Inorganic Chemistry. de Gruyter, Berlin 2007, ISBN 978-3-11-019060-1 , p. 647.
- ^ Brian E. Hanson: The carbon-13 NMR spectrum of solid iron pentacarbonyl. In: Journal of the American Chemical Society. 111, 1989, pp. 6442-6443 ( doi : 10.1021 / ja00198a077 ).
- ^ A b W. Henderson, JS McIndoe: Mass Spectrometry of Inorganic, Coordination and Organometallic Compounds: Tools - Techniques - Tips. John Wiley & Sons Publisher, ISBN 0-470-85015-9 .
- ↑ CPG Butcher, PJ Dyson, BFG Johnson, T. Khimyak, JS McIndoe: Fragmentation of Transition Metal Carbonyl Cluster Anions: Structural Insights from Mass Spectrometry. In: Chemistry - A European Journal . 2003, 9 (4), pp. 944-950 ( doi : 10.1002 / chem . 200390116 ).










![\ mathrm {VCl_3 \ + \ 4 \ Na \ + \ 6 \ CO \ + \ Diglyme \ \ xrightarrow {(200 \ bar, 433 \ K, 48 \ h)} \ Na (digly) _2 [V (CO) _6 ] \ + \ 3 \ NaCl}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f5f7993bf93aa3793befa5340cbf66ddae1bd7f5)
![\ mathrm {2 \ [V (CO) _6] ^ - \ + \ 2 \ H ^ + \ \ longrightarrow \ 2 \ H [V (CO) _6] \ \ longrightarrow \ 2 \ V (CO) _6 \ + \ H_2}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d805c317a8861eed563c81735eb146f67559475a)
![\ mathrm {2 \ Co ^ {2 +} \ + \ 3 \ {S_2O_4 ^ {2 -} \ + \ 12 \ OH ^ - \ + \ 8 \ CO \ longrightarrow \ 2 \ [Co (CO) _4] ^ - \ + \ 6 \ {SO_3} ^ {2 -} \ + \ 6 \ H_2O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/34d938bf59609b29f6d11d3275184bd31354cbb9)


![{\ displaystyle \ mathrm {2 \ [N (C_ {2} H_ {5}) _ {4}] [Ta (CO) _ {6}] \ +2 \ Ag [Al (OC (CF_ {3})) _ {4}] \ longrightarrow \ Ta_ {2} (CO) _ {12} \ +2 \ Ag \ +2 \ [N (C_ {2} H_ {5}) _ {4}] [Al (OC ( CF_ {3}) _ {4}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/70f2c500df1be6f66e7226167f3dae1c40de2d4d)
![\ mathrm {4 \ KCo (CO) _4 \ + \ [Ru (CO) _3Cl_2] _2 \ \ longrightarrow \ 2 \ RuCo_2 (CO) _ {11} \ + \ 4 \ KCl}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8f6b7c98e4224cfd3dcba32d970dbd28f42d87a7)
![\ mathrm {Mn (CO) _5Cl \ + \ AlCl_3 \ + \ CO \ \ longrightarrow \ [Mn (CO) _6] ^ + \ [AlCl_4] ^ -}](https://wikimedia.org/api/rest_v1/media/math/render/svg/177d362e6ea0bcc96a1a59d06dd67d1e77b1db6c)








![\ mathrm {Me (CO) _n \ xrightarrow {{- CO}} \ [Me (CO) _ {n-1}] \ xrightarrow {{+ PPh_3}} \ Me (CO) _ {n-1} PPh_3}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f6b25cf52f5825db4d2561dfff516df015be473a)
![\ mathrm {Me (CO) _n \ xrightarrow {{+ PPh_3}} \ [Me (CO) _nPPh_3] \ xrightarrow {{- CO}} \ Me (CO) _ {n-1} PPh_3}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9085c4f584685973386d65c914e1db20d1e8ce4e)
![\ mathrm {Mn_2 (CO) _ {10} \ + \ 2 \ Na \ \ longrightarrow \ 2 \ Na [Mn (CO) _5]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ecfb565d54ae0af2389987c4c8bb83d490f924ea)
![\ mathrm {(1) \ Fe (CO) _5 \ + \ NaOH \ \ longrightarrow \ Na [Fe (CO) _4COOH] \ \ xrightarrow {{+ NaOH}} \ Na [HFe (CO) _4] \ + \ NaHCO_3 }](https://wikimedia.org/api/rest_v1/media/math/render/svg/ffabccef29d19fcca6ebec2ee72c99bc83788bb7)
![\ mathrm {(2) \ Na [HFe (CO) _4] \ + \ NaOH \ \ longrightarrow \ Na_2 [Fe (CO) _4] \ + \ H_2O}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6f64581546366efcc506c7c20d862d6b24b912fb)
![\ mathrm {Na [HFe (CO) _4] \ + \ H ^ + \ \ longrightarrow \ H_2 [Fe (CO) _4] \ + \ Na ^ +}](https://wikimedia.org/api/rest_v1/media/math/render/svg/28fab82b222f1df4b116024301618c23ce64306a)
![\ mathrm {Mn_2 (CO) _ {10} \ + \ Cl_2 \ \ longrightarrow \ 2 \ [Mn (CO) _4Cl] \ + \ 2 \ CO}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8e9b48dd9a943be3c2a82a96f3e83d282fbc8d82)

![\ mathrm {Na_2 [Fe (CO) _4] + RBr \ \ rightarrow \ Na [RFe (CO) _4] + NaBr}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c17ec033f2db0a43121f950cc3d68de79b055bb8)
![\ mathrm {Na [RFe (CO) _4] + PPh_3 \ \ rightarrow \ Na [R (CO) Fe (CO) _3PPh_3]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e931b224b933cb8afe457a720ca282ded5739058)