Chromium hexacarbonyl
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
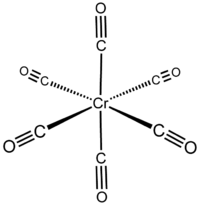
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Chromium hexacarbonyl | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 CrO 6 | |||||||||||||||
| Brief description |
white crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 220.06 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.77 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
149-150 ° C |
|||||||||||||||
| boiling point |
decomposition |
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Chromium hexacarbonyl is a chemical compound with the formula [Cr (CO) 6 ]. Like its molybdenum and tungsten analogues, this colorless compound is a volatile, relatively air-stable complex of chromium in the zero oxidation state.
presentation
Chromium hexacarbonyl is produced by the reduction of chromium (III) chloride (CrCl 3 ) in benzene under carbon monoxide pressure . It succeeds in good yield even in large batches in the autoclave.
It is also possible to use triethylaluminum instead of aluminum and to display it in the case of room pressure using a Grignard connection :
Properties and structure
In the complex, the chromium is octahedral surrounded by six carbonyl ligands (O h ). The dipole moment of the complex is 0 Debye . The Cr-C distance is 191 pm . The wave number of the CO stretching vibration ν CO of the free carbon monoxide is 2000 cm −1 . It is a stable 18-valence electron complex that melts undecomposed at 150 ° C and can decompose explosively when heated rapidly and at temperatures above 210 ° C. The compound can be sublimed without decomposition in a vacuum .
Chromium hexacarbonyl is a colorless, volatile, air-resistant, crystalline and toxic compound. In addition, there is a suspicion of a carcinogenic effect. Like all metal carbonyls, if improperly handled, chromium hexacarbonyl is a source of volatile metal and carbon monoxide. It is insoluble in water and has limited solubility in organic solvents such as diethyl ether and trichloromethane. Its solution is sensitive to light and oxidation.
Reactions
The carbonyl ligands can be split off oxidatively , photolytically or thermally. The resulting free coordination sites can be occupied by solvent molecules . When heated in aromatic solvents, three carbonyl ligands are split off to form a new complex that has one aromatic ligand.
The formation of such complexes is favored for electron-rich aromatics.
Other solvents that are capable of coordination can also coordinate on the complex as labile ligands.
- Cleavage of a carbonyl ligand on heating in tetrahydrofuran
use
Chromium hexacarbonyl can be used to represent Fischer carbenes containing chromium . For this, it is with carbon - nucleophiles such as alkyl lithium implemented, at the carbonyl carbon add . An important application is the Dötz reaction used in the synthesis of aromatic compounds, such as vitamin K or vitamin E has a meaning.
Individual evidence
- ↑ a b c d e f g h i Entry on chromium hexacarbonyl in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ Ernst Otto Fischer, Walter Hafner, Karl Öfele: About aromatic complexes of metals, XXXI. A synthesis for chromium hexacarbonyl , Chemical Reports , Volume 92, Issue 12, pp. 3050-3052, December 1959; doi : 10.1002 / cber.19590921207 .
- ↑ a b Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1818.
- ↑ a b c B. B. Owen et al .: Chromium hexacarbonyl . In: Ludwig F. Audrieth (Ed.): Inorganic Syntheses . tape 3 . McGraw-Hill, Inc., 1950, pp. 156-160 (English).
- ^ A. Whitaker, JW Jeffery: The Crystal Structure of Chromium Hexacarbonyl , Acta Crystallographica , 1967 , 23 , pp. 977-984; doi : 10.1107 / S0365110X67004153 .
- ^ Christoph Elschenbroich : Organometallchemie , 6th edition, Teubner, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , p. 330.

![{\ displaystyle \ mathrm {CrCl_ {3} \ + \ Al \ + \ 6 \ CO \ {\ xrightarrow {\}} \ [Cr (CO) _ {6}] \ + \ AlCl_ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e72d43ddc6c498f4e6b71ca4bf41a4575591a19b)
![{\ displaystyle {\ ce {CrCl3 + C6H5MgBr -> [CO] [] [complex] -> [H_2O] [] Cr (CO) 6}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/22d607251e0647ed37ce493d74c8cc5733532fe4)
![{\ displaystyle \ mathrm {[Cr (CO) _ {6}] + C_ {6} H_ {6} \ longrightarrow [Cr (\ eta ^ {6} -C_ {6} H_ {6}) (CO) _ {3}] + 3 \ CO}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f1cadab045d544e87cea5e96c176d54fab9594e8)
![{\ displaystyle \ mathrm {[Cr (CO) _ {6}] + THF \ longrightarrow [Cr (CO) _ {5} (THF)] + CO}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ed08349103e89ed10cc4f48a8c130b5d75c2415d)