Organic lithium compounds
As organolithium compounds , also organolithium compounds or organolithium compounds are referred to organic compounds that have a direct bond between carbon and lithium own.
Manufacturing
The synthesis of lithium organyls is known as lithiation . Several well-established routes for the synthesis of organolithium compounds are known.
From halides
Alkyl and aryl lithium compounds can be obtained by reacting the corresponding halides with elemental lithium:
- R = organic residue, X = halide.
Suitable halides are chloride , bromide and iodide . Here, however, the Wurtz reaction takes place as a side reaction.
- Wurtz reaction
Instead of elemental lithium, commercially available organolithium compounds can also be used as lithiation reagents. These enter into a metathesis reaction with organohalides , the lithiated compound and the corresponding alkyl halide being formed.
- Lithiation of an aromatic, Ar = aryl, X = halide.
From the cleavage of CH bonds
Another way of lithiation is the deprotonation of the substance to be lithiated with commercially available organolithium compounds, for example n- butyllithium . Here an activated proton is abstracted by the lithium organyl, whereby the desired lithium organyl is formed and the alkane on which the lithium organyl used is based is released.
However, this reaction requires that the lithium organyl formed is less basic than the original. Since the lithium organyl used is strongly basic and consequently reacts with low selectivity, the proton to be abstracted must be significantly more acidic than other protons present in the molecule.
A defined lithiation of aromatics can be achieved, for example, by ortho-directing groups . The ortho -directing groups include, for example, tertiary amines , amides or the methoxy group . These possess free electron pairs that stabilize the lithium ion and thus in close proximity to the ortho -ständigen bring Proton, which can then be abstracted preferred.
This effect is similar to the ortho selectivity, which is used in the Kolbe-Schmitt reaction for the synthesis of salicylic acid .
properties
Due to the high electronegativity difference between lithium (0.98) and carbon (2.55), the lithium-carbon bond has a strongly ionic character. The measured dipole moment is 6 D . In the case of a purely ionic bond, however, a dipole moment of 9.5 D would be expected, which shows the partially covalent character of the bond. The bond is strongly polar, whereby the carbon atom has the negative charge and it has carbanionic properties. However, the ionic part of the bond is in most cases less than the covalent one, so that lithium organyls are not correctly represented as a contact ion pair .
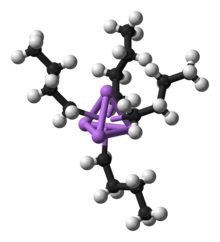
In solution, many lithium organyls are not monomeric , but aggregate into ordered structures. This is due to the coordinative undersaturation of lithium in a 2-electron-2-center bond. For this reason, organolithium compounds often form oligomers in order to achieve coordinate saturation. Thus, n -butyllithium forms tetrameric structures in diethyl ether and hexameric structures in cyclohexane . Structurally, the tetramer is a lithium tetrahedron , on the surfaces of which the alkyl radicals are bound.
All organic lithium compounds are strong bases which sometimes react very violently with water and other protic solvents .
- Reaction of butyllithium with water to form butane and lithium hydroxide .
Some compounds, such as tert -butyllithium , are pyrophoric .
use
For the synthesis of complexes
Lithium is a base metal , which is why bonded residues can be transmetallated onto more noble metals . This property is used, for example, in the production of Schrock carbenes . The residues are transmetallated in the sense of a substitution reaction to the more noble metal ion complexed with unstable ligands , with a lithium salt being obtained as a by-product.

Lithium organyls are also used for the synthesis of Gilman cuprates .
As a nucleophile
The carbanionic character of the carbon atom in lithium organyls enables it to act as a nucleophile . They can be used for addition reactions on electrophiles . For example, lithium organyls add to ketones and aldehydes and enter into substitution reactions with esters .
Aromatic lithium compounds are suitable for introducing a whole series of substituents on the aromatic system. The scope of this reaction begins with simple alkylations , in which mostly an alkyl halide is used. Also bromination , iodination and Carboxylations are possible in this way. Also Boronsäureester as the Suzuki coupling are needed and organotin compounds , for the Stille coupling can be used, are accessible by reaction of the lithium organyl with the corresponding chlorides.
literature
- C. Elschenbroich : Organometallchemie , 6th edition, Vieweg + Teubner Verlag, 2008 , ISBN 3-8351-0167-6 .
- A. Salzer : Organometallic compounds , in: Ullmann's Encyclopedia of Industrial Chemistry 2005 , Wiley-VCH Weinheim.
Individual evidence
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, pp. 543-545, ISBN 3-342-00280-8 .