tert -butyllithium
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
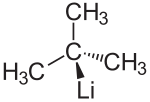
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | tert -butyllithium | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 9 Li | ||||||||||||||||||
| Brief description |
colorless, pyrophoric solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 64.05 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
140 ° C (decomposition) |
||||||||||||||||||
| solubility |
soluble in pentane |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
tert -Butyllithium ( t -BuLi) is a tertiary organometallic compound of the element lithium ( organolithium compound ). There are also the isomeric forms n -butyllithium and sec -butyllithium . The basicity increases in the series n -Butyllithium < sec -Butyllithium < tert -Butyllithium. t -BuLi is thus the strongest base in this series. t -BuLi breaks down into lithium hydride and isobutene at room temperature. The isobutene formed isdeprotonatedby further t -BuLi in the allyl position, which leads to a further reduction in the concentration of t -BuLi.
presentation
The synthesis can be carried out by reacting t -butyl bromide with lithium (powder).
properties
It is only available commercially as a solution in alkanes (mostly in pentane or heptane), a concentration of 1.7 mol / l being common. Ethers such as THF or diethyl ether are unsuitable as solvents for storage because they decompose quickly. t -BuLi is extremely pyrophoric even in solution and burns in the air with a typical red flame .
Reactions
The lithium-carbon bond in the tert- butyllithium molecule is strongly polarized . The carbon atom carries a negative and the lithium atom a positive partial charge. Tert -Butyllithium therefore behaves chemically similar to a corresponding carbanion . This behavior can be illustrated by formulating a mesomeric boundary structure :
Similar to n -Butyllithium , t -BuLi can also be used for lithium - halogen exchange and for the deprotonation of amines and activated CH compounds. t -BuLi can also attack the alpha position of the ethers .
This is shown by the example of tetrahydrofuran , which is decomposed within minutes at room temperature :
This method is used, for example, when deprotonated acetaldehyde is required.
use
In modern synthetic organic chemistry, t -BuLi has achieved importance as an ultra-strong base or as a lithiation reagent. Since it is much more pyrophoric than n -BuLi , it is used less often. In some cases it is used because of the higher basicity or for steric reasons (the t -butyl radical is spatially relatively large).
safety instructions
t -BuLi is extremely pyrophoric even in solution . Handling and storage must therefore always take place under protective gas. t -BuLi also reacts violently with water to form lithium hydroxide and isobutane . With longer storage, a sediment is formed from lithium hydride, which is also pyrophoric (from self-decomposition) and lithium hydroxide (penetrated moisture).
swell
- ↑ Entry on butyllithium. In: Römpp Online . Georg Thieme Verlag, accessed on May 24, 2014.
- ↑ a b Ulrich Wietelmann, Richard Bauer: lithium and lithium compounds. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2005, doi : 10.1002 / 14356007.a15_393 .
- ↑ a b Data sheet tert-butyllithium, nominally 1.5M in n-pentane, packaged under argon in resealable ChemSeal bottles from AlfaAesar, accessed on September 27, 2018 ( PDF )(JavaScript required) .
- ↑ KPC Vollhardt, NE Schore: Organic Chemistry . 3. Edition. Wiley-VCH, 2005, ISBN 978-3-527-29819-8 .








