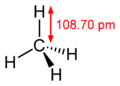Alkanes
In organic chemistry, alkanes ( boundary hydrocarbons , formerly paraffins ) are the group of saturated , acyclic hydrocarbons . That is, their representatives consist only of the two elements carbon (C) and hydrogen (H), have only single bonds and no carbon rings. This makes them a subgroup of the aliphatic hydrocarbons . The general formula C n H 2 n +2 with n = 1, 2, 3, ... applies to them. From n = 4, the basic structure of the alkanes can consist of unbranched (linear) or branched carbon chains. The unbranched compounds are called n- alkanes and form a homologous series of alkanes. The branched alkanes are called isoalkanes ( i- alkanes).
Saturated cyclic hydrocarbons have a different general empirical formula and form the group of cycloalkanes and are described there.
n -alkanes
The simplest alkane is methane . The first twelve n -alkanes are given in the following table. They form the homologous series of alkanes .
| C. | Surname | Molecular formula | Molar mass | Melting point | boiling point | density | Ball and stick model |
|---|---|---|---|---|---|---|---|
| 1 | methane | CH 4 | 16.04 g mol −1 | 90.65 K | 111.4 K | 0.72 kg / m 3 gaseous 0.42 g / cm 3 liquid |
|
| 2 | Ethane | C 2 H 6 | 30.07 g mol −1 | 90 K | 185 K | 1.36 kg / m 3 0.54 g / cm 3 |
|
| 3 | propane | C 3 H 8 | 44.10 g mol −1 | 85 K | 231 K | 2.01 kg / m 3 0.58 g / cm 3 |
|
| 4th | n -Butane | C 4 H 10 | 58.12 g mol −1 | 135 K | 272.5 K | 2.71 kg / m 3 0.60 g / cm 3 |
|
| 5 | n -pentane | C 5 H 12 | 72.15 g mol −1 | 144 K | 309 K | 0.626 g / cm 3 | |
| 6th | n -hexane | C 6 H 14 | 86.18 g mol −1 | 178 K | 342 K | 0.659 g / cm 3 | |
| 7th | n -heptane | C 7 H 16 | 100.2 g mol −1 | 182 K | 371 K | 0.684 g / cm 3 | |
| 8th | n- octane | C 8 H 18 | 114.2 g mol −1 | 216 K | 399 K | 0.703 g / cm 3 | |
| 9 | n -Nonan | C 9 H 20 | 128.3 g mol −1 | 222 K | 424 K | 0.718 g / cm 3 | |
| 10 | n -decane | C 10 H 22 | 142.3 g mol −1 | 243 K | 447 K | 0.73 g / cm 3 | |
| 11 | n -Undecane | C 11 H 24 | 156.3 g mol −1 | 248 K | 469 K | 0.74 g / cm 3 | |
| 12 | n -dodecane | C 12 H 26 | 170.3 g mol −1 | 263 K | 489 K | 0.75 g / cm 3 |
i -alkanes

|

|
| n -Butane | iso- butane (methyl propane) |
With an increasing number of carbon atoms, the number of possibilities for their covalent linkage also increases. That is why all alkanes with a higher number of carbon atoms than propane occur in a large number of constitutional isomers - molecules with the same empirical formula but different structure ( constitution ). These are called isomers .
In the case of butane , the case occurs that with the same empirical formula C 4 H 10, two different arrangements are possible for the carbon atoms in the alkane molecule. Butane exists in two different constitutions: n- butane and iso- butane ( isomeric butane ). The term iso-alkanes - i-alkanes for short - is derived from this.
Pentane already occurs in three different constitutions, the n -alkane with an unbranched chain, the iso- pentane with one branch on the second carbon atom and the neo- pentane with two branches on the second carbon atom.
As the number of carbon atoms increases, so does the number of possible isomers, most of which, however, only exist theoretically - in nature and technology only a few are of importance (see number of isomers of alkanes below). Icosan (formerly Eicosan) with a chain of twenty carbon atoms already has 366,319 different constitutional isomers. For alkanes with 167 carbon atoms, the number of theoretically possible isomers exceeds the estimated number of particles in the visible universe.
Long-chain, branched alkanes are also known as isoparaffins .
Alkane stereochemistry
Branched alkanes can be chiral ; H. With the same constitution different arrangements are possible in mirror image. In the case of 3-methylhexane , for example, this occurs at the carbon in position 3. This is biologically relevant in many biomolecules. The side chain of chlorophyll, like that of tocopherol ( vitamin E ), is a branched chiral alkane. Chiral alkanes can be separated into their enantiomers by enantioselective gas chromatography .
nomenclature
The nomenclature of the alkanes is precisely defined by the International Union of Pure and Applied Chemistry (IUPAC).
All stem names have the ending -an . This ending is preceded by a Greek numeric word that indicates the number of carbon atoms. For the first four alkanes, which are trivial names , the names methane, ethane (previously ethane), propane and butane are instead given for historical reasons. How the names of alkanes with more than ten carbon atoms are formed can be found in the article nomenclature .
The following naming rules apply to branched alkanes:
- The carbon atoms of the longest continuous carbon chain are numbered so that the tertiary or quaternary carbon atoms are each given the lowest possible number. This is the case when the sum of all these numbers is the lowest (example molecule above: 2 + 3 + 4 = 9). The molecule gets its stem name according to this longest chain (example molecule above: 6 carbon atoms → hexane ).
- The names of the branching alkyl groups (side chains) are also determined by their length and are placed in front of the base name of the alkane in alphabetical ascending order (see 4th additional rule a).
- These alkyl group names are preceded by the numbers of the carbon atoms at which they branch off, separated from them by hyphens (see 5th additional rule b).
- Additional rule a) If more than one alkyl group with the same name branches off from the main chain, these alkyl group names are preceded by their number in Greek notation ( di = two, tri = three, etc.) as a numeric word. Please note that these numerical words are not taken into account in alphabetical sorting.
- Additional rule b) If there are several branching alkyl groups with the same name, the numbers are noted in ascending order separated by commas. If two identical alkyl groups branch off from a quaternary carbon atom, the number of the carbon atom is noted twice.
An example for the additional rules a) and b) is 3-ethyl-2,2,4-trimethylhexane: In the 3-ethyl-2,4-dimethylhexane shown above, the hydrogen atom on the second carbon atom would be replaced by a methyl group . Note: The two compounds mentioned have several centers of chirality each , so that this nomenclature is incomplete.
In the past, alkanes were referred to as "limiting hydrocarbons" or paraffins . The latter is derived from the Latin parum affinis , which can be translated as “little related” - it was previously believed that substances that react with each other must have some kind of “relationship” - and thus expressed the relative inertia of these compounds. Today the name usually only refers to a mixture of certain solid alkanes.
Alkyl radical
If a hydrogen atom is withdrawn from an alkane molecule, a radical is created , a molecule with an unbound electron that is known as an alkyl radical. The name of this alkyl radical is obtained by replacing the -an with a -yl at the end of the alkane from which the hydrogen atom has been removed . Symbolically, alkyls are often notated with R ; if the alkyl radicals are different, this is indicated by R 1 , R 2 , R 3 etc.
Molecular geometry
sp³ hybridization with methane .
The spatial structure of the alkanes has a direct effect on their physical and chemical properties. The electron configuration of the carbon is crucial for their understanding. Its atoms have four free electrons in the ground state, the so-called valence electrons , which are available for bonds and reactions. In the unbound carbon atom, these four electrons are in orbitals of different energies, in alkanes, on the other hand, the carbon atom is always sp³-hybridized , which means that four new orbitals of the same energy are present by superimposing the four starting orbitals (one s orbital and three p orbitals ) are. These are spatially arranged in the shape of a tetrahedron , the angle between them is therefore 109.47 degrees.
Bond lengths and bond angles
An alkane molecule has only CH and CC bonds (single carbon bonds). The former arise from the overlap of an sp³ hybrid orbital of carbon with the 1s orbital of hydrogen, the latter from the overlap of two sp³ hybrid orbitals of different carbon atoms.
The bond length is 109 picometers for the CH bond and 154 picometers for the CC bond, so the distance between two carbon atoms is about 50 percent greater than the distance between a carbon and a hydrogen atom, which is primarily due to the different atomic radii .
The spatial arrangement of the bonds results from the alignment of the four sp³ orbitals - since these are arranged in a tetrahedral manner, these are also the CC and CH bonds, so there is a fixed angle of 109.47 degrees between them. The structural formula , which shows the bonds of the molecules in a completely straight line, does not correspond to reality in this respect.
Conformation of the alkanes
Knowledge of the structural formula and the bond angle does not usually fully determine the spatial structure of a molecule. For every carbon-carbon bond there is a further degree of freedom : the angle that the three atoms or groups of atoms bound to the two bonding atoms make with respect to one another. The spatial arrangement described by these angles is called the conformation of the respective molecule.
Ethane
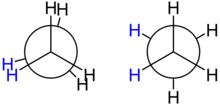
The simplest case within the alkane class is ethane; there is exactly one CC bond here. If you look at the molecule along the defined axis, you get what is known as the Newman projection : one carbon atom can be seen in the projection with its three hydrogen atoms in the foreground, the other is symbolically covered by a circle and is by definition in the background ; the bonds to its three hydrogen atoms can only be partially seen in the diagram. Both the front and the rear three hydrogen atoms take a 120 degree angle to one another in the projection, as must also apply to the projection of a tetrahedron into the plane. However, the angle θ between the two groups of hydrogen atoms is not fixed - it describes the conformation in the ethane molecule.
The conformation angle can assume any value between 0 and 360 degrees, but qualitatively only two different conformations are of interest:
- In the ecliptic conformation , the conformation angle is 0, 120 or 240 degrees; in the projection, a front and a rear hydrogen atom coincide.
- In the staggered conformation , the conformation angle is 60, 180 or 300 degrees, so that in the projection a rear hydrogen atom comes to lie between two front ones.
The two conformations, also called rotamers , differ in their energy, which in this case is referred to as the torsional energy, by about 12.6 kilojoules per mole. While the ecliptic conformation maximizes this and is therefore unstable, it is minimized by the staggered conformation , this is therefore energetically preferred. All other conformations lie between these two extremes in terms of their energy. The cause of the difference has not yet been fully clarified: In the ecliptic conformation, the distance between the CH bond electrons of the front and rear carbon atoms is smaller, the electrostatic repulsion between them and thus the energy of the state is consequently higher. Conversely, the staggered conformation allows greater delocalization of the binding electrons, a quantum mechanical phenomenon that stabilizes the structure and reduces the energy. Today, the latter explanation is considered more likely.
The torsional energy of the ethane molecule is small at room temperature compared to the thermal energy, so that it is then in constant rotation around the CC axis. However, it "locks" in the staggered conformation at regular intervals, so that around 99 percent of all molecules are close to the energy minimum at any point in time. The transition between two adjacent staggered conformations only takes an average of 10 −11 seconds.
Higher alkanes
While qualitatively the same applies to the two CC bonds of the propane molecule as to ethane, the situation for butane and all higher alkanes is more complex.
If one looks at the mean CC bond of the butane molecule, each of the two carbon atoms is bonded to two hydrogen atoms and one methyl group. As can be seen from the Newman projection, four qualitatively different rotamers can be distinguished, between which any transition states are possible. As with ethane, they correspond to conformations of maximum or minimum energy:
- If the two methyl groups are at the same point in the projection, i.e. at a torsion angle of 0 degrees, one speaks of the fully ecliptic or synperiplanar conformation. It corresponds to a global maximum of the torsional energy, since the hydrogen atoms of the methyl groups come so close that there is a repulsion between their electron clouds.
- At a torsion angle of 60 or 300 degrees, the conformation is called skewed or synclinar ; In contrast to the ecliptic structures, all atoms or groups of atoms of the front carbon atom come to lie between those of the rear carbon atom in the projection. This results in a minimum of energy; Due to the proximity of the two methyl groups to one another, however, it is only local, so there is still an energetically more favorable conformation.
- If the torsion angle is 120 or 240 degrees, the conformation is partial-ecliptic . It is energetically unfavorable for the same reasons as with ethane. In contrast to the fully ecliptic conformation, the two methyl groups do not come too close, so that the energy only represents a maximum locally.
- Finally, at a torsion angle of 180 degrees, there is an antiperiplanar conformation. As with the staggered conformation of ethane, an increased delocalization of the electrons , which can only be explained by quantum mechanics, occurs , while the two methyl groups occupy the greatest possible spatial distance from one another. The torsional energy is therefore minimized globally for this state.
The energy gap between the syn- and antiperiplanar conformation is about 19 kilojoules per mole and is therefore still small compared to the thermal energy at room temperature. Therefore rotations around the central CC axis are still easily possible. As with ethane, however, the probability of finding a molecule in a certain state is not the same; for the antiperiplanar conformation it is about twice as high as for the synclinal conformation, while it is negligibly small for the two ecliptic conformations.
With the higher alkanes, the picture is basically the same - for all CC bonds the antiperiplanar conformation, in which the attached alkyl groups occupy the greatest possible distance, is the most favorable in terms of energy and therefore the most likely to be encountered. For this reason, the structure of the alkanes is usually represented by a zigzag arrangement; this is also the case in the molecular models shown above. The actual structure will always differ somewhat from this idealized conformation at room temperature - so alkane molecules do not have a fixed rod shape, as the model might suggest.
Other hydrocarbon molecules, such as the alkenes, are based on this basic structure, but also contain stiffened sections due to double bonds , which can lead to permanent "bending".
The chains of the higher alkanes basically have an elongated conformation. From 18 to 19 chain links, folded hairpin structures can also be detected at low temperatures . To form hairpin structures, the hydrocarbon chains must first be heated and then cooled extremely quickly to minus 150 ° C using a carrier gas.
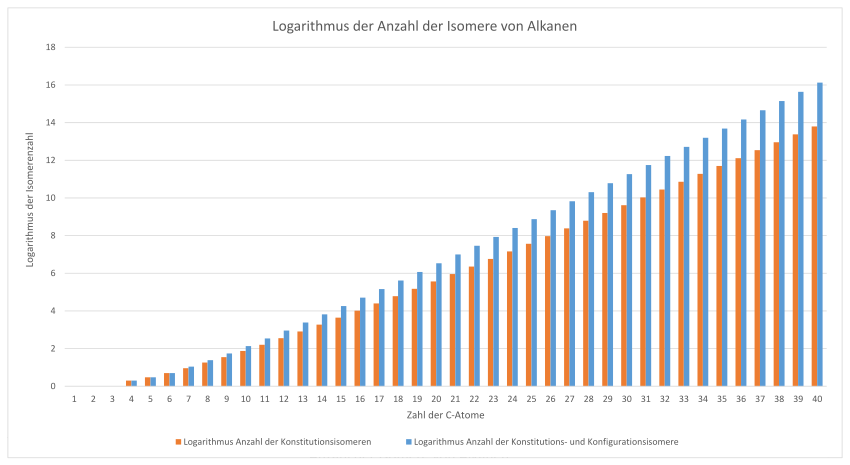
Number of isomers of alkanes
There is no simple formula for determining the number of isomers of alkanes; you have to work with an algorithm and a tree structure.
| Number of carbon atoms | Number of constitutional isomers | Number of constitution and
Configurational isomers |
|---|---|---|
| 1 | 1 | 1 |
| 2 | 1 | 1 |
| 3 | 1 | 1 |
| 4th | 2 | 2 |
| 5 | 3 | 3 |
| 6th | 5 | 5 |
| 7th | 9 | 11 |
| 8th | 18th | 24 |
| 9 | 35 | 55 |
| 10 | 75 | 136 |
| 11 | 159 | 345 |
| 12 | 355 | 900 |
| 13 | 802 | 2,412 |
| 14th | 1,858 | 6,563 |
| 15th | 4,347 | 18,127 |
| 16 | 10,359 | 5.0699 |
| 17th | 24,894 | 143.255 |
| 18th | 60,523 | 408.429 |
| 19th | 148.284 | 1,173,770 |
| 20th | 366.319 | 3,396,844 |
| 21st | 910.726 | 9,892,302 |
| 22nd | 2,278,658 | 28,972,080 |
| 23 | 5,731,580 | 85.289.390 |
| 24 | 14,490,245 | 252.260.276 |
| 25th | 36,797,588 | 749.329.719 |
| 26th | 93.839.412 | 2,234,695,030 |
| 27 | 240.215.803 | 6,688,893,605 |
| 28 | 617.105.614 | 20,089,296,554 |
| 29 | 1,590,507,121 | 60.526.543.480 |
| 30th | 4,111,846,763 | 182.896.187.256 |
| 31 | 10,660,307,791 | 554.188.210.352 |
| 32 | 27,711,253,769 | 1,683,557,607,211 |
| 33 | 72.214.088.660 | 5,126,819,371,356 |
| 34 | 188.626.236.139 | 15.647.855.317.080 |
| 35 | 49.378.2952.902 | 47,862,049,187,447 |
| 36 | 1,295,297,588,128 | 146.691.564.302.648 |
| 37 | 3,404,490,780,161 | 450,451,875,783,866 |
| 38 | 8,964,747,474,595 | 1,385,724,615,285,949 |
| 39 | 23,647,478,933,969 | |
| 40 | 62.481.801.147.341 |
These numbers are the theoretically possible isomers. However, many of them are not viable for steric reasons. This list shows the number of constitutional isomers up to 100 carbon atoms.
properties
Alkanes form a particularly uniform class of substances; knowledge of the properties of a few representatives is sufficient to predict the behavior of the others. This applies both to the intra- and intermolecular interactions of the alkanes, which affect the melting and boiling points , as well as to the consideration of their syntheses and reactions .
Physical Properties
The molecular structure, especially the size of the surface of the molecules, determines the boiling point of the associated substance: the smaller the area, the lower the boiling point, since the Van der Waals forces acting between the molecules are smaller; a reduction of the surface can be achieved by branching or by a ring-shaped structure. In practice this means that alkanes with a higher carbon content usually have a higher boiling point than alkanes with a lower carbon content; unbranched alkanes have higher boiling points than branched ones and ring-shaped ones have higher boiling points than unbranched ones. From five carbon atoms, unbranched alkanes are liquid under normal conditions, from seventeen they are solid. The boiling point increases by between 20 and 30 ° C per CH 2 group.
With two exceptions for ethane and propane, the melting point of the alkanes also rises when the number of carbon atoms increases; however, especially in the case of the higher alkanes, the melting points rise more slowly than the boiling points. In addition, the melting point of alkanes with an odd carbon number to alkanes with an even carbon number increases more than vice versa. The cause of this phenomenon is the greater packing density of alkanes with an even carbon number. The melting point of the branched alkanes can be either above or below the corresponding value for the unbranched alkanes. The more bulky the molecule, the more difficult it is to pack the relevant substance tightly and, consequently, the lower the melting point. Conversely, there are a number of isoalkanes which have a much more compact structure than the corresponding n -alkanes; in this case, therefore, their melting points are above those of their straight isomers.
Alkanes neither conduct electricity nor are they permanently electrically polarized . For this reason they do not form hydrogen bonds and are very difficult to dissolve in polar solvents such as water . Since the hydrogen bonds between the individual water molecules in the immediate vicinity of an alkane point away from it and are therefore not isotropically aligned, i.e. do not point evenly in all directions, a mixture of both substances would be associated with an increase in molecular order. Since this is forbidden according to the second law of thermodynamics , two separate layers always form when attempting to mix. Alkanes are therefore called water-repellent or hydrophobic . Their solubility in non-polar solvents, on the other hand, is good, a fact that is referred to as lipophilic . They can be mixed with one another in any ratio, for example, given the same aggregate state.
With a few exceptions, the density of the alkanes increases with the number of carbon atoms. Since it is lower than that of water for all liquid alkanes, alkanes always float on top if you try to mix them, which is why burning, liquid alkanes cannot be extinguished with water.
Chemical properties
In general, alkanes show a relatively low reactivity because their CH and CC bonds are relatively stable and cannot easily be broken. Unlike most other organic compounds, they also have no functional groups .
They only react very poorly with ionic or more generally polar substances. Their p K s value is above 60 (methane 48), so they practically do not react with normal acids or bases, which is also indicated by the common name paraffin (Latin: parum affinis = slightly inclined). In crude oil, the alkane molecules have even remained chemically unchanged for millions of years.
However, alkanes enter into redox reactions , especially with oxygen and the halogens, since their carbon atoms are in a greatly reduced state; in the case of methane, even the lowest possible oxidation state −IV is achieved. In the first case it is a question of burns , in the second case of substitution reactions .
Radicals , i.e. molecules with unpaired electrons, play a major role in most reactions, including what is known as cracking and reforming, in which long-chain alkanes are converted into short-chain ones and unbranched ones into branched ones.
In the case of strongly branched molecules, there is a deviation from the optimal bond angle, which is caused by the fact that alkyl groups on different carbon atoms would otherwise come too close in space. The resulting “tension”, known as steric tension , makes these molecules much more reactive.
Reactions
Reactions with oxygen
All alkanes react with oxygen and are therefore flammable but not fire-promoting ; however, their flash point rises as the number of carbon atoms increases. Compared to other hydrocarbons such as alkenes and alkynes , they react to release most of the energy. A double or triple bond releases more energy than a single bond, but this is overcompensated by the higher number of oxidizable hydrogen atoms in the molecule (6 for ethane, 4 for ethene and 2 for ethyne). The standard enthalpies of combustion are approximately at the following values:
- Ethane : Δ c H ° = −1560.7 kJ / mol
- Ethene : Δ c H ° = −1411.2 kJ / mol
- Ethyne : Δ c H ° = −1301.1 kJ / mol
Thus, most of the energy is released when the ethane is burned (negative enthalpy). With sufficient oxygen supply, alkanes burn with a weakly luminous, non-sooting flame.
Chemically, the reaction with oxygen is a redox reaction in which the alkanes are oxidized and the oxygen is reduced. When completely burned, the carbon reacts to carbon dioxide (oxidation number + IV) and the hydrogen to water, which is released in the form of water vapor:
- An n -alkane reacts with oxygen to form water and carbon dioxide.
The total combustion energy increases comparatively regularly with an increasing number of carbon atoms; each CH 2 group contributes about 650 kilojoules per mole. From the fact that the combustion energy of branched alkanes is somewhat lower than that of unbranched alkanes, one can conclude that the former group is more stable.
If alkanes are not completely burned because too little oxygen is available, undesired by-products such as alkenes, carbon and carbon monoxide are formed and the energy yield is lower; a complete combustion of the alkanes is therefore important. An example of incomplete combustion is the following reaction
- Octane reacts with oxygen to form carbon dioxide, carbon monoxide , carbon (soot), propene and water.
Black smoke therefore indicates insufficient oxygen supply when burning gasoline.
Reactions with the halogens
Another important reaction group of the alkanes are halogenation reactions - they too belong to the larger group of redox reactions, as the oxidation numbers of the carbon atoms concerned change.
In halogenation, the hydrogen atoms of an alkane are partially or completely replaced or substituted by halogen atoms such as fluorine , chlorine or bromine , which is why one speaks of a substitution reaction . The reaction produces so-called haloalkanes , usually in a mixture, as can be seen from the following example for methane:
- Methane reacts with chlorine to form mono-, di-, tri- and tetrachloromethane as well as hydrogen chloride .
The mixing ratio of the individual haloalkanes depends on the reaction conditions and the course of the reaction and is chosen completely arbitrarily in the reaction equation, i.e. not representative.
The reaction with chlorine is triggered even with low energy input in the form of ultraviolet light - the high yield of the reaction per unit of energy indicates that it is a chain reaction . In this case, once a halogen radical is present, a new one is constantly reproduced as the reaction proceeds, at least until the excess of halogen atoms has been broken down. So this is a radical substitution .
As with any chain reaction, there are three steps involved in halogenation reactions:
- Initiation: The diatomic halogen molecules are split homolytically, for example, by high-energy light irradiation, i.e. with a symmetrical division of the electrons, into radicals:
- Propagation Step 1: A halogen radical releases a hydrogen atom from an alkane molecule and leaves an alkyl radical behind:
- Propagation Step 2: An alkyl radical releases a halogen atom from a halogen molecule and leaves a halogen radical behind:
- Step 1 and step 2 alternate continuously during the reaction - an increasing number of reaction products are formed from a few initial chlorine radicals.
- Termination: The reaction stops when the probability of two radicals encountering one another is greater than that of encountering a starting product (or an alkane that is not yet fully halogenated). In this case, recombination occurs:
- Although the last of these termination reactions also leads to haloalkanes, the number of products produced in this way is negligible compared to the number of those produced in the chain reaction.
In the above consideration, no statement was made as to which hydrogen atoms are replaced first in the case of a given alkane. This question does not arise for the most important cases of methane or ethane, since all hydrogen atoms are equivalent. From propane onwards, however, some of them are bound to secondary or tertiary carbon atoms, i.e. those with two or three bonds to other carbon atoms. These bonds are weaker, which in the case of halogenation has the effect that the hydrogen atoms located on a secondary or even tertiary carbon are preferably replaced by halogens.
Example: 2-chloropropane CH 3 –CHCl – CH 3 occurs more frequently as a reaction product than 1-chloropropane CH 2 Cl – CH 2 –CH 3 than would be statistically expected.
The reaction rates are extremely different for the four halogens. At 27 ° C the ratio is
F: Cl: Br: I = 140,000: 1300: 9 · 10 −8 : 2 · 10 −19 .
From this the different course of the reactions can already be read off: With fluorine the alkanes react in a barely controllable way, with chlorine moderately, with bromine weakly and only under the influence of light, with iodine, however, practically not at all.
Iodination is even energetically unfavorable, which is why iodine is used as a radical scavenger in halogenation reactions in order to stop the chain reactions. By stopping the substitution reaction, this can be partially controlled in order to increase the yield of a particular reaction product.
Chlorinated and fluorinated methane gases are of particular technical importance, but the reaction to them can lead to an explosion . Trichloromethane was previously used as an anesthetic under the name of chloroform , fluorine-chlorine hydrocarbons served as propellants for a long time until they lost their importance due to their harmful effects on the earth's ozone layer .
Haloalkanes are chemically detectable with the help of the Beilstein test .
Cracking and reforming
Important reactions in the processing of crude oil are cracking and reforming .
In the former, the much more sought-after lower alkanes are obtained from higher alkanes under high pressure and at high temperature . C [BOND] C bonds are split on catalysts such as aluminum oxide . example
- Dodecane splits into a decyl and an ethyl radical.
These recombine to form new alkanes. Example:
- An ethyl and a propyl radical react to form pentane.
Suitable reaction conditions can be used to ensure that primarily short alkane molecules are formed as a result of these reactions. In the same way, disruptive side reactions such as the formation of alkenes can largely be prevented. Usually hydrogen is added during cracking in order to remove impurities such as sulfur or nitrogen - this is called hydrocracking .
Reforming, on the other hand, is necessary with alkane mixtures that are to be used as gasoline. For this purpose, unbranched alkanes, which have unfavorable combustion properties for this purpose, are converted into branched alkanes and arenes , i.e. aromatic hydrocarbons, on catalysts .
More reactions
With the help of nickel catalysts, hydrogen can be obtained from the alkanes in a reaction with water vapor. Further reactions of the alkanes are sulfochlorination and nitration , a reaction with nitric acid, but both of which require special conditions. Fermentation to alkanoic acids is technically far more important .
hazards
Methane can explode from an air volume fraction of one to eight percent without a source of ignition and is a powerful greenhouse gas , and some other mixtures of lower alkanes and air are self-igniting from a certain alkane fraction. Due to their flammability, alkanes can pose a hazard; however, as the carbon content increases, so does the flash point. Propane, butane, pentane, hexane, heptane and octane and some other alkanes are hazardous substances, but their MAK value is set relatively high, for pentane it is 3000 mg / m³, for hexane only 180 mg / m³. Pentane, hexane, heptane and octane are also dangerous to the environment and higher alkanes are mostly classified as irritants or no longer hazardous substances at all .
presentation
The low carbon alkanes can be made from the elements themselves; higher alkanes must be produced under high pressure using the Bergius process . Some of the reactions have been given their own names .
The representation of alkanes can be done in several ways:
- via catalytic hydrogenation of alkenes , for example according to
- Preparation from haloalkanes with the aid of hydrogen . Example:
- Tetrachloromethane reacts with hydrogen to form hydrogen chloride and methane
- via the Kolbe electrolysis ; in this case alkanecarboxylates are reduced to the radical, which breaks down into alkyl radicals with release of carbon dioxide , these dimerize to alkanes.
- via the Wurtz synthesis , in which alkanes are formed with the formation of metal halides from haloalkanes and metal organyls. General reaction equation:
- Haloalkanes react with metal organyls to form alkanes and metal halogens.
- Via the Bergius process , the alkanes are produced under high pressure from coal and hydrogen.
- Via the Fischer-Tropsch process , liquid alkanes are produced from carbon monoxide and hydrogen . Example:
- Carbon monoxide reacts with hydrogen to form methane and water .
In 1985 one of the longest alkanes ever synthesized was prepared; it consists of molecules with a chain length of exactly 390 carbon atoms (C 390 H 782 ).
Occurrence
Alkanes occur both on earth and in the solar system , but only about the first 100, most of them only in traces. Of great importance on other celestial bodies are primarily the light hydrocarbons: The two gases methane and ethane could be detected both in the tail of the comet Hyakutake and in some meteorites , the so-called carbonaceous chondrites . They also form an important part of the atmospheres of the outer gas planets Jupiter , Saturn , Uranus and Neptune . For a long time, entire oceans of these and longer-chain alkanes were suspected on Saturn's moon Titan , but today it is assumed that there are at most smaller lakes made of ethane. On Mars , traces of methane were discovered in the atmosphere, which is the strongest evidence of living beings (soil bacteria) on that planet to date.
On earth, methane occurs in traces in the atmosphere, the content is around 0.0001 percent or 1 ppm ("parts per million") and is primarily produced by the bacterial archaea . The content in the oceans is negligible due to the lack of solubility in water, but methane is found under high pressure and at low temperature in water ice frozen at the bottom of the oceans as so-called methane hydrate . Although it cannot be commercially mined to this day, the calorific value of the known methane hydrate fields exceeds the energy content of all natural gas and crude oil deposits combined many times over - methane obtained from methane hydrate is therefore a candidate for future fuels.
However, the most important commercial sources of alkanes today are clearly natural gas and petroleum , which are the only organic compounds naturally occurring in mineral form . Natural gas primarily contains methane and ethane, as well as propane and butane, while crude oil consists of a mixture of liquid alkanes and other hydrocarbons. Both emerged when dead marine animals were covered by sediments in the absence of oxygen and were converted into their respective natural substances over the course of many millions of years at high temperatures and high pressure. Natural gas was created through the following reaction, for example:
- Dextrose reacts to methane and carbon dioxide under high pressure and temperature.
They then collected in porous rocks, which were sealed at the top by impermeable layers. In contrast to methane, which is constantly being re-formed on a large scale, higher alkanes do not arise in nature to any significant extent. Their resources will therefore be exhausted in a few decades.
Solid alkanes occur as evaporation residues of petroleum that has emerged, known as earth wax . One of the largest deposits of natural solid alkanes is in the so-called asphalt lake of La Brea on the Caribbean island of Trinidad .
Use and further processing
On the one hand, alkanes are important raw materials in the chemical industry , where they are processed into plastics , for example , and, on the other hand, they are the most important fuels in the global economy.
The starting point for processing is always natural gas and oil. The latter is separated in the oil refinery by fractional distillation and then processed into many other important products such as gasoline . This takes advantage of the fact that different “fractions” of the crude oil have different boiling points and can thus be easily separated from one another. In contrast, the boiling points within the individual fractions are close together.
The respective area of application of a certain alkane can be divided quite well according to the number of carbon atoms it contains, although the following delimitation is idealized and does not strictly apply:
The first four alkanes are mainly used for heating and cooking purposes. Methane and ethane are the main components of natural gas; they are normally stored under pressure in a gaseous state. However, their transport is cheaper in the liquid state, the gas must then be compressed by high pressure for this purpose.
Propane and butane can be liquefied by contrast, have low pressure and therefore come in liquid before being used as fuel - as LPG in internal combustion engines and in agriculture when driving tractors. Propane is used, for example, in propane gas burners, butane in lighters - when it escapes, the slightly pressurized liquid, which consists of 95 percent n- butane and 5 percent isobutane, turns into a mixture of gas and fine droplets and is so easy to ignite. In addition, the two alkanes are used as propellants in spray cans.
Pentane to octane are highly volatile liquids and can therefore be used as fuel in normal internal combustion engines, since they easily change into the gaseous state when they enter the combustion chamber and do not form droplets there, which would impair the uniformity of combustion. Only branched alkanes are desirable in fuel because they do not tend to pre-ignite like the unbranched ones. A measure of the pre-ignition of a type of gasoline is its octane number . It indicates the extent to which a substance tends to spontaneously ignite. The two alkanes heptane ( n- heptane) and iso- octane (2,2,4-trimethylpentane) were arbitrarily chosen as reference numbers for the octane values, each having an octane number of 0 (heptane, tends to pre-ignition) and an octane number of 100 ( iso - Octane, hardly tends to spontaneously ignite). The octane number of a fuel indicates how much volume% iso- octane in a mixture of iso- octane and heptane corresponds to its knocking properties. In addition to their function as fuel, the middle alkanes are also good solvents for non-polar substances.
Alkanes from nonane to about hexadecane, an alkane with sixteen carbon atoms, are liquids of higher viscosity , so they are more viscous and are therefore less and less suitable for use in ordinary gasoline as the carbon number increases. Instead, they form the main component of diesel fuel and aviation fuel . Since the mode of operation of a diesel engine or a turbine is fundamentally different from that of a gasoline engine, their greater viscosity does not matter here. However, because of its high content of long-chain alkanes, diesel fuel can solidify at low temperatures, a problem that mainly arises in areas near the poles. After all, the specified alkanes are part of petroleum and were previously used in petroleum lamps.
Alkanes from hexadecane upwards are the most important components of heating oil and lubricating oil . In the latter function, they also act as anti- corrosion agents , as their hydrophobic nature means that no water can get to the parts at risk of corrosion. Many solid alkanes are used as paraffin wax , which can be used to make candles , for example . However, it should not be confused with real wax , which consists primarily of esters .
Alkanes with a chain length of around 35 or more carbon atoms are found in asphalt , so they are used, among other things, as road surfaces. Overall, however, the higher alkanes are of little importance and are therefore mostly broken down into lower alkanes by cracking.
Alkanes in living nature
Alkanes occur in many ways in nature, but are not biologically essential substances .
Alkanes in bacteria and archaea
Certain types of bacteria convert alkanes in their metabolism . Even-numbered carbon chains are preferred because they are more easily degradable than odd-numbered ones.
Conversely, some archaea , the so-called methane generators , produce large quantities of the lightest alkane, methane, from carbon dioxide. They obtain the energy required for this through the oxidation of molecular hydrogen:
- Carbon dioxide reacts with hydrogen to form methane and water.
Methane formers are also the producers of the swamp gas released in bogs and swamps, which is produced in a similar way in the digestion towers of sewage treatment plants, and releases around two billion tons of methane every year - the atmospheric content of this gas is almost exclusively produced by them.
The methane emissions of the cellulose- digesting herbivores, u. a. the ruminants - especially the cattle , which can release up to 150 liters per day - to the termites is ultimately due to methane producers. On a - albeit smaller - scale, they also produce this simplest of all alkanes in the human intestine. Methane-forming archaea are therefore crucially involved in the carbon cycle by returning photosynthetically bound carbon to the atmosphere. Today's natural gas deposits are likely to be largely due to this group of living beings.
Alkanes in fungi and plants
In the three main eukaryotic groups of living things, fungi , plants and animals , alkanes also play a certain role, albeit a minor one overall. In the former, the more volatile representatives appear mainly in the spores ; some specialized yeasts , the alkane yeasts , also use alkanes as a source of energy and carbon. The kerosene mushroom ( Amorphotheca resinae ) preferentially metabolizes aviation fuel from long-chain n- alkanes.
In the case of plants, in addition to branched, cyclic, unsaturated and organic substances containing heterocomponents, the long-chain solid representatives are primarily found; Together with the other connections, they form a solid wax layer in almost all of them, which covers the outer skin exposed in the air, the cuticle . Their function is on the one hand to protect against dehydration, on the other hand to prevent important minerals from being washed out by rain and, finally, to ward off bacteria, fungi and insect pests - the latter often sink with their legs into the soft, waxy substance and become as a result when running with special needs. The shiny layer on fruits such as apples also consists of long-chain alkanes. The carbon chains are usually between twenty and forty atoms long and have alkanoic acids as precursors in plants' wax synthesis. Since the alkanoic acids are built up from C 2 units (citrate cycle) and alkanes are formed by loss of the carboxy group - decarboxylation - leaf wax alkanes of higher land plants have an odd carbon number preference in the above-mentioned carbon number range. The exact composition of the wax layer is not only dependent on the species, it also changes with the season and also depends on environmental factors such as light conditions, temperature or humidity. However, it was found that especially grasses, most clearly grasses of the tropical and subtropical vegetation zones (in steppes and savannas ), are clearly distinguished in the chain length distribution patterns of the alkanes by a slight offset to longer carbon chains compared to trees and bushes. Use this fact z. B. Agronomists in the nutritional research of herbivores , as well as climate researchers to assess the climate-dependent grass distribution of the earth in the geological past.
Alkanes in animals
In animals, alkanes occur in oily tissues, but in contrast to unsaturated hydrocarbons, they do not play a significant role there. An example is the shark liver, can be obtained from the an oil which was about 14 percent of pristane is, an alkane having the structure name 2,6,10,14-tetramethyl pentadecane (C 19 H 40 ). More important is their occurrence in pheromones , chemical messenger substances that insects in particular depend on for communication. In some species, such as the longhorn beetle Xylotrechus colonus , which mainly produces n-pentacosane (C 25 H 52 ), 3-methylpentacosane (C 26 H 54 ) and 9-methylpentacosane (C 26 H 54 ), they are transmitted through body contact, so that one speaks of contact pheromones. Also in others such as the Tse-Tse fly Glossina morsitans morsitans , whose pheromone is primarily composed of the four alkanes 2-methylheptadecane (C 18 H 38 ), 17,21-dimethylheptatriacontane (C 39 H 80 ), 15,19-dimethylheptatriacontane (C 39 H 80 ) and 15,19,23-trimethylheptatriacontane (C 40 H 82 ), the substances act through body contact and serve as a sexual attractant - a fact that is used to combat this disease vector.
Alkanes and ecological relationships
An example in which both the vegetable and animal use of alkane play a role is the ecological interrelationship between the species Andrena nigroaenea , which belongs to the sand bees ( Andrena ), and the great spider ragwort ( Ophrys sphegodes ), which belongs to the orchids (Orchidaceae ). . The latter is dependent on the former for pollination . Sand bees also use pheromones to find partners; In the case of Andrena nigroaenea , the females of the species use a mixture that consists of tricosan (C 23 H 48 ), pentacosan (C 25 H 52 ) and heptacosan (C 27 H 56 ) in a ratio of 3: 3: 1 - becoming males attracted by exactly this fragrance. The orchid takes advantage of this fact - parts of its flower not only look like sand bees, but also emit large amounts of the above three substances - in the same ratio. Even the wax covering the leaves has the same chemical composition as the sexual attractant of female bees. As a result, numerous males are attracted to the flowers and carry out so-called pseudo - populations there , thus attempting to reproduce on the flower with an imaginary partner. While this undertaking is naturally unsuccessful for the bees, the attempts at copulation transfer pollen to the respective insect, which after frustrated withdrawal can be brought to other flowers, i.e. used to reproduce the orchid. The use of alkanes, known as chemical mimicry , enables the spider ragwur to largely dispense with the energy-intensive production of conventional insect attractants.
literature
- Peter W. Atkins : Short textbook physical chemistry. Wiley-VCH, Weinheim 2001, ISBN 3-527-30433-9 .
- Peter Pfeifer, Roland Reichelt (Eds.): H 2 O & Co Organic Chemistry. Oldenbourg, Munich 2003, ISBN 3-486-16032-X .
- KPC Vollhardt, Neil E. Schore: Organic Chemistry. Wiley-VCH, Weinheim 2000, ISBN 3-527-29819-3 .
- Eberhard Breitmaier, Günther Jung: Organic chemistry. Thieme, Stuttgart 2001, ISBN 3-13-541504-X .
- F. Rommerskirchen, A. Plader, G. Eglinton, Y. Chikaraishi, J. Rullkötter: Chemotaxonomic significance of distribution and stable carbon isotopic composition of long-chain alkanes and alkan-1-ols in C 4 grass waxes. In: Organic Geochemistry , 37, 2006, pp. 1303-1332, doi: 10.1016 / j.orggeochem.2005.12.013 .
Web links
- Encyclopedia: structure, relationships, occurrence, use, properties and structure, cracking
- Properties, occurrence, nomenclature and isomers
- Explanations suitable for students
Individual evidence
- ↑ Entry on alkanes . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.A00222 .
- ↑ Der Brockhaus, Science and Technology , Mannheim; Spectrum Academic Publishing House, Heidelberg 2003.
- ^ Uwe Meierhenrich : Amino Acids and the Asymmetry of Life , Springer-Verlag, Heidelberg / Berlin 2008, ISBN 978-3-540-76885-2 .
- ^ Nils OB Lüttschwager, Tobias N. Wassermann, Ricardo A. Mata, Martin A. Suhm: The last alkane with an extended ground state conformation . In: Angewandte Chemie . tape 124 , no. 39 , 2012, p. 482-485 , doi : 10.1002 / anie.201202894 .
- ^ Structural isomers of alkanes , script from the University of Bayreuth , accessed on July 21, 2017.
- ^ Siegfried Hauptmann, Jürgen Graefe, Horst Remane: Textbook of Organic Chemistry . VEB German publishing house for basic industry, Leipzig 1976. DNB 770047483
- ↑ List of stereoisomers of alkanes ( OEIS list A000628 ) Retrieved on July 23, 2017
- ↑ Number of constitutional isomers up to 100 C atoms. Accessed July 22, 2017.
- ^ Arnold Arni: Grundkurs Chemie I and II , Wiley-VCH, 2011, ISBN 978-3-527-33068-3 , pp. 18-19.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Heat of Combustion, pp. 5-70.
- ↑ Entry for CAS no. 109-66-0 in the GESTIS substance database of the IFA , accessed on June 29, 2015(JavaScript required) .
- ↑ Entry for CAS no. 110-54-3 in the GESTIS substance database of the IFA , accessed on June 29, 2015(JavaScript required) .
- ↑ DA Carlson, PA Langley, P. Huyton: Sex pheromone of the tsetse fly: isolation, identification, and synthesis of contact aphrodisiacs. In: Science 201, 1978. pp. 750-753; doi: 10.1126 / science.675256 .
- ^ M. Ayasse, W. Francke, BS Hansson, F. Ibarra, C. Löfstedt, HF Paulus, FP Schiestl: Orchid pollination by sexual swindle. In: Nature 399, 1999. p. 421; doi: 10.1038 / 20829 .