Halogenation
In chemistry, halogenation is the conversion of an element or a compound into a halide , a salt-like or covalent compound with a halogen . This is possible for both inorganic and organic compounds. Depending on the halogen, a distinction is made between fluorination , chlorination , bromination or iodination .
Addition reactions
Halogen, hydrogen halide or hypohalous acid react with unsaturated compounds.
Addition of halogens to alkenes or alkynes
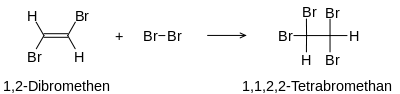
Alkenes react with halogen molecules to form vicinal dihaloalkanes. Alkynes add halogen gradually. Tetrahaloalkanes are formed. Shown here using the example of the reaction of Br 2 with ethyne:
The first step is the formation of the vicinal dihaloalkene (here 1,2-dibromoethene ).
In the second step, the tetrahaloalkane is produced (here 1,1,2,2-tetrabromoethane).
Addition of hydrogen halide to alkenes or alkynes
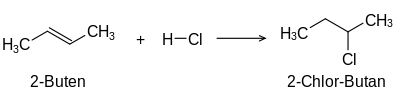
The addition of hydrogen halide to an alkene produces a monohaloalkane. Alkynes react with hydrogen halide to form a monohalogenalkene, the halogen atom being added to one of the two sp 2 -hybridized carbon atom orbitals of the carbon-carbon double bond. An example of the addition of hydrogen halides to alkenes is the addition of hydrogen chloride to 2-butene :
Addition of hypohalous acid to alkenes
The addition of hypohalous acid to alkenes yields halohydrins , ie both sp 2 -hybridized carbon atoms in the educt are converted into sp 3 -hybridized carbon atoms in the product in which a hydroxyl radical is bonded to one of these carbon atoms and a halogen atom to the other.
Addition of halogens to free radicals
Because of the easy homolytic cleavage of halogen molecules, they react spontaneously with free organic radicals.
Substitution reactions
Halogenation of aromatics
A variant of halogenating aromatics is electrophilic aromatic substitution . Activated halides attack the aromatic system electrophilically . An example of this is the chlorination of benzene :
If chlorine is allowed to react with benzene in the presence of a Lewis acid , chlorobenzene and hydrogen chloride are formed . Iron (III) chloride or aluminum trichloride mostly functions as a Lewis acid and is used to activate chlorine, which would otherwise not react with benzene. The reaction with bromine would proceed analogously.
Halogenation of alkanes
The halogenation of alkanes is free radical and leads to haloalkanes .
- Radical substitution of ethane with Cl 2
Halogenation in the allyl position or benzyl position
The halogenation of alkenes in the allyl position or of alkyl aromatics in the benzyl position takes place radically and, with substitution, leads to halogenated alkenes or alkyl aromatics halogenated in the side chain :
Gross reaction using the example of cyclohexene .
Halogenation of ketones and aldehydes
The halogenation of ketones is formally an electrophilic substitution reaction with a proton as a leaving group. However, it does not proceed according to such a mechanism, but via the enol of the ketone in question and α-halogenated ketones are formed. The halogenation of aldehydes proceeds analogously.
Mechanism of halogenation of aldehydes and ketones
The halogenation of carbonyl groups , such as those present on aldehydes and ketones, can be either acid -catalyzed or base-catalyzed . In the following, the mechanism is demonstrated using an acid-catalytic bromination of acetone:

Mechanism of Halogenation of Carbonyl Compounds; R denotes a hydrogen atom for an aldehyde and an organic radical for a ketone.
The acetone ( 1 ) used is in equilibrium with its enol form via the keto-enol tautomerism . If bromine is now added, it attaches itself to the enol form as described and the oxonium ion 2 is formed , which has a further mesomeric boundary structure. In addition, a bromine ion is formed, which deprotonates the oxonium ion 2 in the following step . This simply gives brominated acetone 3 . If these steps are repeated twice for this brominated acetone 3 , a triple brominated acetone 4 can be obtained. One then speaks of an α, α, α-trihalogenated acetone.
The rate-limiting step in this reaction is the formation of the enol. The rate of reaction decreases with each halogen added to the molecule, since the halogens are electron withdrawing. This makes the rearrangement of electrons in the molecule to the enol form more difficult. It is also said that the enolizability of the compound was lowered.
Halogenation of carboxylic acids
Carboxylic acids can be converted into carboxylic acid chlorides by reaction with thionyl chloride . Other chlorinating agents such as B. phosphorus trichloride or phosphorus pentachloride can also be used.
Halogenation of ethers
Ethers can be chlorinated at low temperatures to form α-chloro and α, α'-dichloro ethers .
Substitution of hydroxyl groups
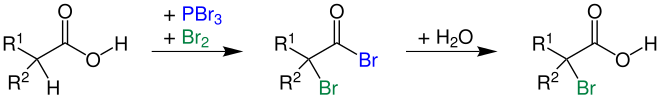
Hydroxy groups in alcohols can be substituted by chlorine using hydrogen chloride or by bromine using hydrogen bromide. For primary and secondary alcohols in particular, thionyl chloride (SOCl 2 ) is often used for chlorination or phosphorus tribromide (PBr 3 ) for bromination. In order to replace hydroxyl groups with iodine, in addition to hydrogen iodide, phosphorus triiodide is also used in the laboratory, which has to be produced in situ from phosphorus and iodine.
Primary and secondary alcohols can also be chlorinated or brominated using the Appel reaction . The reaction is stereoselective , but inverses the configuration .
Substitution of oxygen from carbonyl groups
When aldehydes or ketones are heated with phosphorus trichloride or phosphorus tribromide, geminal dichlorides or dibromides are formed.
Substitution of nitrogen-containing groups by halogen
Reaction of diazonium salts with the formation of organic halogen compounds. In addition to the Sandmeyer reaction, the Balz-Schiemann reaction is important here.
Halogenation on the nitrogen atom of carboxamides
Carboxamides which have at least one hydrogen atom on the amide nitrogen atom react with hypohalites. It produced N -Halogencarbonsäureamide. One example is N- bromo succinimide (NBS), which is obtained in an aqueous solution from succinimide and one equivalent each of a base and bromine .
Halogenation in Inorganic Chemistry
In inorganic chemistry, halogenation plays a role in the formation of salts , hydrogen halides and oxygen halides, among other things .
literature
- Marye Anne Fox, James K. Whitesell: Organic Chemistry, Fundamentals, Mechanisms, Bioorganic Applications , Spectrum, Akad. Verl., 1995, ISBN 3-86025-249-6 .
Individual evidence
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 517.
- ^ Hans Beyer and Wolfgang Walter : Organic Chemistry , S. Hirzel Verlag, Stuttgart, 1984, p. 73, ISBN 3-7776-0406-2 .
- ^ Siegfried Hauptmann : Reaction and Mechanism in Organic Chemistry , BG Teubner, Stuttgart, 1991, p. 113, ISBN 3-519-03515-4 .
- ^ K. Peter C. Vollhardt, Neil E. Schore, "Organic Chemistry", Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, 4th edition, H. Butenschön, ISBN 3-527-31380-X , p 779-783.
- ↑ Paula Yurkanis Bruice: Organic Chemistry. 4th edition, Prentice-Hall, 2003, ISBN 0-13-141010-5 , pp. 607-609.
- ^ Ivan Ernest: Binding, Structure and Reaction Mechanisms in Organic Chemistry , Springer-Verlag, 1972, pp. 297–306, ISBN 3-211-81060-9 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 240, ISBN 3-342-00280-8 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 297, ISBN 3-342-00280-8 .
- ↑ Siegfried Hauptmann : Reaction and Mechanism in Organic Chemistry , BG Teubner, Stuttgart, 1991, pp. 95–96 and p. 136, ISBN 3-519-03515-4 .
- ↑ a b c K. Peter C. Vollhardt, Neil E. Schore: Organic Chemistry , Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, 4th edition, H. Butenschön, ISBN 3-527-31380-X , Pp. 921-922.
- ↑ Paula Yurkanis Bruice: Organic Chemistry. 4th edition, Prentice-Hall, 2003, ISBN 0-13-141010-5 , p. 797.
- ^ Hans Beyer and Wolfgang Walter : Organische Chemie , S. Hirzel Verlag, Stuttgart, 1984, pages 238-239, ISBN 3-7776-0406-2 .
- ↑ a b Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 518.
- ^ Substitution reactions on aliphatics . EPF Lausanne, accessed on November 2, 2011.
- ↑ a b Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 519.
- ^ Allinger , Cava , de Jongh , Johnson , Lebel , Stevens : Organic Chemistry , Walter de Gruyter & Co., 1980, p. 898, ISBN 3-11-004594-X .