Hell-Volhard-Zelinsky reaction
The Hell-Volhard-Zelinsky reaction or also Zelinsky reaction is a name reaction in organic chemistry . In this reaction, named after Carl Magnus von Hell (1849–1926) (published 1881), Jacob Volhard and Nikolaj Dimitrievic Zelinskij (extension of the reaction 1887), a hydrogen atom on the α-carbon atom of a carboxylic acid is replaced by a halogen (mostly bromine ).
Overview reaction
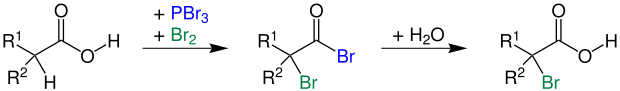
The carboxylic acid is mixed with a halogen and a catalytic amount of a phosphorus halide . An α-halocarboxylic acid is formed. If you z. B. bromine and catalytic amounts of PBr 3 are used, one receives an α-bromocarboxylic acid (2-bromocarboxylic acid):
mechanism
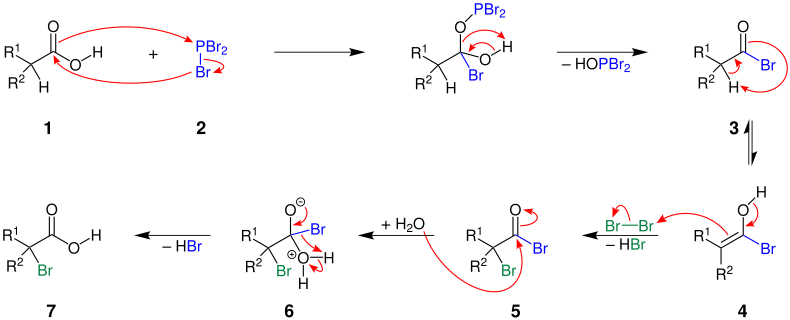
The mechanism is illustrated here using the reaction of a carboxylic acid with phosphorus (III) bromide and bromine:
The carboxylic acid 1 reacts with phosphorus tribromide ( 2 ) via an intermediate step to form a carboxylic acid bromide 3 . This tautomerizes to the enol form 4 . In the reaction with bromine, an α-bromocarboxylic acid bromide 5 is formed with elimination of hydrogen bromide . The carbonyl group in 5 is now attacked by a water molecule. The α-bromocarboxylic acid 7 is formed via intermediate stage 6 - hydrogen bromide being split off.
Applications
Various derivatives can be produced from α-halocarboxylic acid:
- α- hydroxy acids by reacting with sodium hydroxide
- α- amino acids through a reaction with ammonia
- Malonic acid derivatives by reaction with sodium cyanide and subsequent hydrolysis of the nitrile
See also
literature
- H. Beyer , W. Walter : Textbook of organic chemistry . 23. revised and updated edition, S. Hirzel Verlag, Stuttgart / Leipzig 1998, ISBN 3-7776-0808-4 .
Web links
- Entry in www.organische-chemie.ch
Individual evidence
- ↑ a b c Z. Wang: Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 1371 f.
- ↑ C. Hell : About a new method of bromination of organic acids . In: Ber. German Chem. Ges. Volume 14 , 1881, p. 891-893 , doi : 10.1002 / cber.188101401187 .
- ↑ J. Volhard : About representation of α-brominated acids . In: Justus Liebigs Ann. Chem. Band 242 , no. 1-2 , 1887, pp. 141-163 , doi : 10.1002 / jlac.18872420107 .
- ↑ N. Zelinsky : About a convenient way of presenting α-bromopropionic ester . In: Ber. German Chem. Ges. Volume 20 , no. 1 , 1887, p. 2026 , doi : 10.1002 / cber.188702001452 .
- ↑ Michael B. Smith: March's Advanced Organic Chemistry . Wiley & Sons, 7th edition, 2013, ISBN 978-0-470-46259-1 , pp. 672-673.
- ↑ L. Kürti , B. Czakó: Stratigic Applications of Named Reactions in Organic Synthesis . Elsevier Academic Press, Amsterdam 2005, ISBN 978-0-12-429785-2 , p. 200.
- ↑ T. Laue, A. Plagens: Name and catchword reactions of organic chemistry . Teubner Verlag, 2006, ISBN 3-8351-0091-2 , p. 182-184 .