Aluminum chloride
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| _Al 3+ _Cl - | |||||||||||||||||||
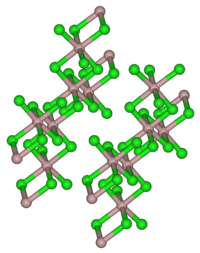
| Crystal system |
monoclinic |
||||||||||||||||||
| Space group |
C 2 / m (No. 12) |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Aluminum chloride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | AlCl 3 | ||||||||||||||||||
| Brief description |
white to yellowish solid with a pungent odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 133.34 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
2.44 g cm −3 |
||||||||||||||||||
| Sublimation point |
180 ° C (262 ° C decomposition) |
||||||||||||||||||
| solubility |
good in water (450 g l −1 at 20 ° C, decomposition) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−704 kJ mol −1 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Aluminum chloride is an inorganic chemical compound; it is the chloride of aluminum with the empirical formula AlCl 3 .
synthesis
Hydrous aluminum chloride ( hexahydrate AlCl 3 · 6H 2 O occurring in rhombic crystals ) is formed by dissolving aluminum in hydrochloric acid :
However, this hexahydrate cannot be dehydrated because it decomposes to aluminum hydroxide or aluminum metahydroxide and hydrogen chloride gas when heated :
Anhydrous aluminum chloride must be produced by passing chlorine over carbon and aluminum oxide at around 800 ° C or directly from the elements:
respectively:
For the large-scale production of aluminum chloride, enamelled stirred tanks are used due to the high level of aggressiveness of the reactants involved.
properties
Aluminum chloride forms colorless, hexagonal crystals ( monoclinic crystal structure, space group C 2 / m (space group No. 12) , a = 5.914 Å , b = 10.234 Å, c = 6.148 Å, β = 108.25 °). It is soluble in many organic solvents. The powder, which is mostly light yellow due to contamination with iron chlorides, has a very hygroscopic effect . In moist air it smokes because of partial hydrolysis to hydrogen chloride and aluminum oxychloride. It dissolves in water under intense warming to form the hexahydrate . In non-polar solvents , in the liquid phase and in the vapor state, aluminum chloride is present as a dimer (Al 2 Cl 6 ) in which the aluminum atom is tetrahedrally coordinated (analogous to aluminum bromide ). In the solid state there is an ion lattice in which the aluminum ion is coordinated 6-fold by Cl - . When melting, the ion lattice breaks down to form the dimer. Since this has a covalent structure, liquid aluminum chloride is a poor conductor of electricity.
The bonding relationships in aluminum (III) chloride are classified as a borderline case between covalent and ionic bonding, it has an electronegativity difference ΔEN of 1.55 (according to Pauling). So it should actually be a polar atomic bond .
Responsiveness
In a strongly exothermic reaction, aluminum chloride dissolves in water , with hydrolysis in chloride ions and hexaaqua aluminum complexes :
These hexaaqua aluminum ions act as a weak acid (p K s = 4.97):
The hydrate cannot be dehydrated by heating to form anhydrous aluminum chloride, since the release of water and hydrogen chloride results in the aluminum hydroxide or aluminum oxide.
use
The hexahydrate of aluminum chloride is used because of its strong astringent effect in the textile and soap industry, where it is used, among other things, in the manufacture of antiseptic agents or deodorants . It also acts as a strong Lewis acid and in organic synthesis (mostly anhydrous here) as a catalyst in dehydrogenation , polymerisation and Friedel-Crafts reactions ( Friedel-Crafts alkylation , Friedel-Crafts acylation ). It is also used as a halogen carrier and condensing agent.
Solutions containing aluminum chloride or aluminum chlorate are offered to gargle against slight inflammation in the throat . It is available over the counter in pharmacies and drug stores.
Aluminum chloride is used in thin-layer chromatography in the form of a spray reagent to detect flavonoids . For this, 2.0 g of aluminum chloride hexahydrate are dissolved in 100 mL of a 5% solution (V / V) of glacial acetic acid in methanol . After this solution has been sprayed onto the TLC plate, it is viewed in the UV 365 light. This reagent is listed under the name aluminum chloride reagent R in the European Pharmacopoeia (Ph. Eur.).
safety instructions
In 2014, aluminum chloride was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of aluminum chloride were concerns about exposure of workers , high (aggregated) tonnage and high risk characterization ratio (RCR) as well as the hazards arising from a possible assignment to the group of CMR substances. The re-evaluation has been running since 2015 and is carried out by France . In order to be able to reach a final assessment, further information was requested.
Commercial preparations
AHC20 (CH), AHC30 (CH), Everdry (D), Gargarisma zum Gurgeln (D), Mallebrin (D), Never-Sweat (D), Odaban (GB), Seven days (D), Sweat Protect (D) , Yerka (D), Purax (D)
See also
Individual evidence
- ↑ Entry on aluminum chloride. In: Römpp Online . Georg Thieme Verlag, accessed on November 11, 2014.
- ↑ a b c d e f data sheet aluminum chloride (PDF) from Merck , accessed on February 4, 2017.
- ↑ entry to aluminum chloride in the GESTIS Bank of IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Entry on aluminum chloride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ PAETEC Formula Collection Edition 2003, p. 116.
- ↑ SI Troyanov: Crystal structures of titanium tetrachloroaluminates Ti (AlCl 4 ) 2 and refinement of AlCl 3 structure crystal. In: Russian Journal of Inorganic Chemistry. 37, 1992, pp. 266-272.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements Wiley-VCH, 1990, ISBN 3-527-26169-9 .
- ^ AF Hollemann, N. Wiberg: Textbook of inorganic chemistry . 102nd edition. De Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1158 .
- ↑ G. Jander, E. Blasius: Textbook of analytical and preparative inorganic chemistry. 12th edition. S. Hirzel Verlag, Leipzig 1982, p. 275.
- ^ AF Hollemann, N. Wiberg: Textbook of inorganic chemistry . 102nd edition. De Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1152 .
- ^ E. Merck AG (Ed.): Staining reagents for thin-layer and paper chromatography . Darmstadt 1965, p. 1 .
- ↑ European Pharmacopoeia . 4.00 edition. tape 1 . Deutscher Apotheker Verlag / Govi-Verlag - Pharmazeutischer Verlag GmbH, Stuttgart / Eschborn 2002, ISBN 3-7692-2947-9 , p. 375 .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Aluminum chloride , accessed on March 26, 2019.







![{\ displaystyle {\ ce {AlCl3 + 6H2O -> [Al (H2O) 6] ^ {3 +} {} + 3Cl-}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7fa18725451127cd374cc0e67c39c572d35f1c95)
![{\ displaystyle {\ ce {[Al (H2O) 6] ^ {3 +} {} + H2O -> [Al (H_ {2} O) _ {5} OH] ^ {2 +} {} + H3O +} }}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5d462c22a0409097f3104c2dea610676039b789b)
![{\ displaystyle {\ ce {[Al (H2O) 6] Cl3 -> Al (OH) 3 + 3HCl + 3H2O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a16f12d8111c10611ecd33c5381c22618a8b5fb9)
![{\ displaystyle {\ ce {2 [Al (H2O) 6] Cl3 -> Al2O3 + 6HCl + 9H2O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/73ec7c6511903fd86ee2ba49910a19cb289adbb6)
