Exothermic reaction
A chemical reaction is exothermic when it releases more energy than was initially supplied as activation energy . (The name comes from the Greek ἔξω exo 'outside' and θερμός thermós 'warm', 'hot', 'heated').
The products of an exothermic reaction have a lower enthalpy than the starting materials ; the enthalpy of reaction of an exothermic reaction is therefore negative.
If the reaction takes place at constant pressure (i.e. under isobaric conditions), the decrease in enthalpy is numerically equal to the amount of heat given off (see → Enthalpy for a more detailed explanation). In the isobaric case, the exothermic reactions are precisely those in which heat is given off to the environment.
Occasionally, exothermic reactions are also defined across the board as reactions that give off heat. In the isobaric case, both definitions are identical, but generally not beyond that. If the reaction takes place, for example, with the volume kept constant, the amount of heat given off corresponds to the change in the internal energy of the system, not the change in enthalpy (see → Enthalpy for a more detailed explanation). This article uses the definition used at the beginning as a reaction with negative enthalpy of reaction. This definition has the advantage that the enthalpy is a state variable , so knowledge of the initial and final state is sufficient to determine the enthalpy change. The heat given off, on the other hand, is a process variable and it is generally necessary to know details about the course of the process in order to be able to calculate it.
The opposite of the exothermic reaction is the endothermic reaction , whose enthalpy of reaction is positive and which in the isobaric case absorbs the amount of heat corresponding to the increase in enthalpy. If a reaction under consideration is exothermic, then the reverse reaction is endothermic, and vice versa.
If the reaction takes place in an adiabatic container so that no heat can be exchanged with the environment, an exothermic reaction leads to an increase in temperature and an endothermic reaction to a decrease in temperature.
In physics, a nuclear reaction that releases energy is called exothermic. An exothermic nuclear fusion is the burning of hydrogen , as happens in the sun . The nuclear fission of uranium, for example, is also strongly exothermic .
Examples
Typical exothermic reactions are:
- Fire ( burning )
- Setting (= hardening) of concrete .
- After brief heating to react iron and sulfur under light and heat development to iron sulfide
The mixing of substances ( heat of mixing ) or the adsorption and absorption of substances, for example on activated carbon or zeolites , is often exothermic, albeit to a much lesser extent .
Exothermic and exergonic reactions

At first glance, it seems reasonable to assume that the exothermic reactions are precisely those reactions that take place voluntarily, and that the more heat is released, the more violent they are. In many cases, the chemical reactions actually behave that way. This experience led to the formulation of the principle by Thomsen and Berthelot in the early years of thermochemistry . This empirical - but not strictly valid - rule states: If reactants are brought together under isobaric and isothermal conditions so that a chemical reaction can take place, then the resulting new equilibrium state is characterized in that the process leading to it releases more heat than any other possible process Process. In other words: Of all possible processes, the most exothermic is realized. The principle is also synonymous with the statement that the implemented process makes the enthalpy difference as large as possible and thus the resulting enthalpy as small as possible.
The existence of voluntary expiring endo Thermer reactions (eg, an evaporating liquid) shows, however, that this principle can not claim universal validity. The actual criterion is: precisely those reactions take place voluntarily which lead to an increase in the total entropy of the system and its surroundings. Under isobaric and isothermal conditions, this criterion of total entropy maximization is synonymous with minimizing the free energy of the system. A reaction that reduces the Gibbs energy of the system is called an exergonic reaction. The distinction between voluntary and non-voluntary reactions is synonymous with the distinction between exergonic and endergonic reactions .
An example of an endothermic, but nonetheless voluntary chemical reaction is the breakdown of nitrous oxide into nitrogen monoxide and nitrogen dioxide :
The enthalpy of reaction of this decay is positive, so the reaction is endothermic. The Gibbs reaction energy is negative, however, so the reaction is exergonic.
The change in Gibbs energy is under isothermal conditions
- .
At low temperatures and the minimization of the Gibbs energy is approximately equivalent to the minimization of the enthalpy of the system. In this case the exergonic reactions are mostly also exothermic reactions, and the principle of Thomsen and Berthelot predicts the states of equilibrium approximately correctly by considering the change in enthalpy. Even at higher temperatures (such as room temperature), the principle remains approximately correct, since the temperature dependencies of and at temperatures that are not too high are similar (as can be shown taking into account the third law ) and the similarity of and therefore with a temperature increase also over a larger one Temperature range is maintained.
However, if a reaction is accompanied by a sufficiently large increase in entropy (as in the aforementioned cases of the evaporating liquid or the decomposition of nitrous oxide), then it can happen that the term predominates and the reaction takes place voluntarily (exergonic, ), although its enthalpy increases (endothermic, ), the reaction in terms of enthalpy is "uphill".
procedure
Even if a reaction is exergonic, i.e. it takes place voluntarily from an energetic point of view, this does not guarantee that it will start to take place independently even without external impetus. An example of this is the reaction of carbon with oxygen to form carbon dioxide :
This combustion reaction is exergonic ( ). However, a lump of coal does not catch fire on mere contact with the oxygen in the air; it has to be ignited first. The combustion then continues independently.
In such cases the reactants are not originally in a reactive state. Often bonds have to be broken before they can be re-formed in a new arrangement - in this case those of the molecule. Usually this happens when two atoms or molecules involved collide. During the interaction during the collision, the original bonds are first stretched, for which energy has to be expended. The colliding particles are briefly present as a so-called activated complex , the enthalpy content of which is greater than that of the individual particles combined; the enthalpy usually comes from the thermal movement of the particles. If the activated complex has enough enthalpy to completely break the relevant bonds (the so-called activation enthalpy), then the reaction can take place. Otherwise the reaction does not take place, although it would be thermodynamically possible - it is “kinetically inhibited” - and the reactants remain in their metastable initial state. In this case, the reaction can only be started if sufficient additional energy is supplied to the system, usually in the form of thermal energy.
In the case of an exothermic reaction, this thermal energy can come from the reaction itself. If the reaction releases enough enthalpy, it is self-sustaining once started.
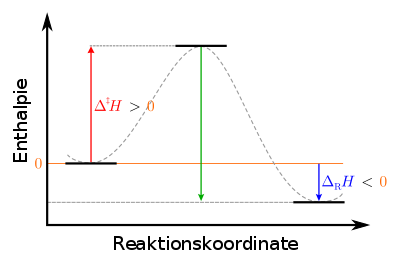
|
Legend : left: initial state of the starting materials: metastable, center: transition state of the activated complex: unstable right: final state of the products: stable |
For the endothermic reverse reaction, the activation enthalpy to be applied is greater than for the forward reaction, namely the sum of the activation enthalpy and reaction enthalpy of the forward reaction, as can also be seen from the diagram.
Nuclear physics
The term exothermic is also used for nuclear reactions . It states that energy is released during the reaction, which occurs as the kinetic energy of the reaction products. Technically important exothermic nuclear reactions are the neutron-induced nuclear fission and the nuclear fusion of hydrogen isotopes.
Individual evidence
- ↑ Entry on exothermic reaction . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.E02269 Version: 2.3.3.
- ^ PW Atkins: Physical Chemistry. 2nd reprint d. 1st edition. VCH, Weinheim 1990, ISBN 3-527-25913-9 , p. 85: "Reactions in which ΔH > 0 are called endothermic, reactions with ΔH <0 are called exothermic."
- ↑ E. Keszei: Chemical Thermodynamics. Springer, Berlin / Heidelberg 2012, ISBN 978-3-642-19863-2 , p. 222: "[...] for exothermal reactions (for which Δ r H ° is negative) [...] for endothermic reactions ( for which Δ r H ° is positive) [...] "
- ↑ a b Entry on enthalpy. In: Römpp Online . Georg Thieme Verlag, accessed on July 11, 2017.
- ↑ PW Atkins, J. de Paula: Physical chemistry. 5th edition, Wiley-VCH, Weinheim 2013, ISBN 978-3-527-33247-2 , p. 46: “A process that releases energy in the form of heat is called exothermic. [...] Processes that have to be supplied with thermal energy are called endothermic. "
- ^ PW Atkins: Physical Chemistry. 2nd reprint d. 1st edition. VCH, Weinheim 1990, ISBN 3-527-25913-9 , p. 85
- ↑ HB Callen: Thermodynamics and an Introduction to Thermostatistics. 2nd Edition. John Wiley & Sons, New York 1985, ISBN 0-471-86256-8 , p. 277.
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. Walter de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 53 (Reading sample: Part A - Basics of the chemistry of hydrogen. Google book search ). .
- ↑ HB Callen: Thermodynamics and an Introduction to Thermostatistics. 2nd Edition. John Wiley & Sons, New York 1985, ISBN 0-471-86256-8 , p. 278.
- ^ PW Atkins: Physical Chemistry. 2nd reprint d. 1st edition. VCH, Weinheim 1990, ISBN 3-527-25913-9 , p. 860.
- ^ PW Atkins: Physical Chemistry. 2nd reprint d. 1st edition. VCH, Weinheim 1990, ISBN 3-527-25913-9 , p. 764.
- ↑ L. Pauling: General Chemistry. Dover, New York 1988, ISBN 0-486-65622-5 , p. 567.






















