Endothermic reaction
As endothermic be in the chemistry called reactions in which energy is supplied must. The standard enthalpy difference is positive. The enthalpy H is the sum of the internal energy of a system and the product of pressure and volume. It is the heat content of a system at constant pressure. Referred to the difference between the energies of the end ( ) and raw materials ( ), so the energy absorbed, applies to endothermic reactions .
An endothermic reaction is therefore a reaction in which energy, for example in the form of heat, is absorbed from the environment. It is the opposite of an exothermic reaction . An example of an endothermic reaction is photosynthesis which takes place in plants .
Also, the melting of ice or evaporation of water is endothermic, but is not a chemical reaction , but a physical change in the physical appearance .
Course of an endothermic reaction
As with exothermic reactions, endothermic reactions also take place in two steps. First, a certain activation energy has to be applied, then part of this energy is released again. The difference to the exothermic reaction is that the energy released is less than the activation energy and is therefore not sufficient to drive the reaction further. The reaction energy is positive. Therefore, in order not to interrupt the reaction, energy must be continuously supplied from outside during the reaction.
So that an endothermic reaction can take place at all (is exergonic ), the reaction must be favored by an increase in entropy and thus have a negative free enthalpy . Endothermic reactions therefore often take place at high temperatures, since, according to the Gibbs-Helmholtz equation, the entropy component of the free enthalpy is greater at these . This can be seen, for example, in the Boudouard equilibrium , in which the endothermic reaction to carbon monoxide takes place at high temperatures .
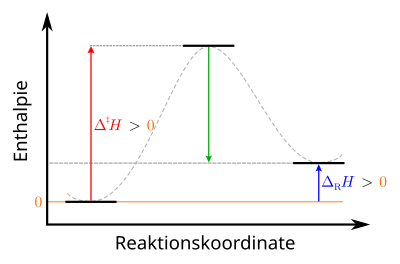
|
Legend : left: initial state of the starting materials: stable center: transition state of the activated complex: unstable right: final state of the products: metastable |
For example: Carrying out a coke layer water vapor, is an endothermic reaction takes place: .
Industrial chemistry
As allotherm endothermic reactions in industrial chemistry are called. Important examples of this are allothermal pyrolysis , in which the biomass is split by externally supplied heat, or steam reforming in the production of synthesis gas . The contrast to this are autothermal reactions in industrial parlance .
physics
In physics , a nuclear reaction is called endothermic if it can only take place with an external supply of energy. The energy can be present as the kinetic energy of the initial reactants that they have received, for example, in a particle accelerator . Another way of supplying energy is heating to a high temperature z. B. in thermonuclear fusion .
Web links
Individual evidence
- ↑ Entry on endothermic reaction . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.E02095 Version: 2.3.2.
- ↑ entry to enthalpy, H . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.E02141 Version: 2.3.2.
- ^ Klaus Weissermel , Hans-Jürgen Arpe : Industrial organic chemistry: Significant preliminary and intermediate products. Wiley-VCH, 2007, ISBN 978-3-527-31540-6 .








