Boudouard equilibrium
| Temperature in ° C | CO 2 in% | CO in% |
| 450 | 98 | 2 |
| 600 | 77 | 23 |
| 700 | 42 | 58 |
| 800 | 6th | 94 |
| 900 | 3 | 97 |
| 1000 | 1 | 99 |
| Boudouard equilibrium at 10 5 Pa | ||
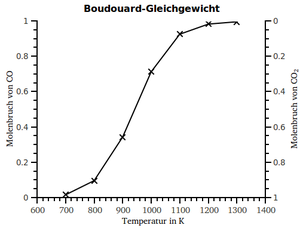
The Boudouard equilibrium is the equilibrium between carbon dioxide (CO 2 ) and carbon monoxide (CO), named after Octave Leopold Boudouard (1872–1923 ), which is established when reacting with glowing carbon .
The enthalpy of formation of carbon dioxide is −393.5 kJ / mol. During the reduction with carbon, two moles of carbon monoxide are formed ( = −110.5 kJ / mol), the process in the gas phase is strongly endothermic (calculated +172.5 kJ / mol).
Due to the endothermic reaction, high temperatures shift the equilibrium to the product side (CO), an increase in pressure shifts it to the reactant side , as the number of gaseous molecules decreases as a result. (See principle of least compulsion ). At room temperature the rate of conversion becomes immeasurably small: carbon monoxide is metastable.
The reaction is used in the production of generator gas and represents an important sub-process in the smelting of iron ore in the blast furnace .
See also
Web links
- Video: Calculating the BOUDOUARD equilibrium for different temperatures . Jakob Günter Lauth (SciFox) 2013, made available by the Technical Information Library (TIB), doi : 10.5446 / 15706 .
swell
- ↑ David R. Lide: CRC Handbook of Chemistry and Physics . 90th edition. Taylor & Francis, 2009, ISBN 978-1-4200-9084-0 .
- ↑ Reference data for enthalpies of education can be found in the CRC Handbook of Chemistry and Physics : STANDARD THERMODYNAMIC PROPERTIES OF CHEMICAL SUBSTANCES ( Memento from February 25, 2015 in the Internet Archive ) (Chapter 5.4) as well as Benson's method

