Benson method
The Benson method is a method for estimating the enthalpy of formation , entropy of formation and heat capacity of substances in the ideal gas phase . The method was developed by the American chemist Sidney Benson (1918–2011) in 1958, later further optimized with modern computer programs and modified for other physical states. The method found its way into modern textbooks as early as 1976.
principle
The method is based on the assumption that each atom of a molecule a specific contribution to the thermodynamic properties of a molecule contributes . The bond types of all atoms must be known in principle for this, spatial geometries ( structural isomers , conformers / rotamers and symmetries) only lead to smaller special contributions in the accumulation of the increments (individual contributions).
Systematics using the example of methanol
Methanol consists of a methyl group and a hydroxyl group , the increments to be used for this are designated C - (H 3 ) (O) and O - (H) (C).
- To calculate the enthalpy of formation in the gas phase (298 K), the two increments −42.26 and −159.33 kJ / mol are to be used (methanol calc. −201.59; exp. −201.10 kJ / mol).
- To calculate the molar heat capacity C p , the increments 25.73 and 18.16 J / mol K are used (methanol calc. 43.89, exp. 43.89 J / mol K)
- To calculate the entropy , in addition to the increments 127.32 and 121.50, a symmetry contribution of −9.13 J / mol K must be taken into account (methanol calc. 239.69, exp. 239.70 J / mol K).
The work by Domalski (1993) also allows the calculation of thermodynamic data for the liquid and solid aggregate state with other incremental sets.
application
The real benefit lies in being able to calculate these thermodynamic quantities for any molecule. Heats of reaction (endothermic / exothermic) in chemical reactions can be easily estimated from the differences in the enthalpies of formation of all reactants, for example in the case of the oxidation of methanol. With consistent use of data for the gaseous or liquid state, computationally comparable reaction enthalpies are obtained ( Hess's heat law ) >> Not only is the method fairly easy to apply, but it usually can estimate properties with an uncertainty no larger than typical experimental uncertainties. << (N. Cohen 1996)
Reference data for standard enthalpies of education can be found in the CRC Handbook of Chemistry and Physics in Chapter 5.4. Values for the gas state and the liquid state differ by definition by the amount of the standard enthalpy of vaporization (25 ° C).
Enthalpy of reaction oxidation of methanol
The enthalpy of formation of methanol is −201.6 kJ / mol. During the oxidation formaldehyde ( = −108.6 kJ / mol) and water ( = −241.5 kJ / mol) are formed, the process in the gas phase is strongly exothermic (calc. −148 kJ / mol).
Enthalpy of reaction hydration of formaldehyde
If the formaldehyde gas ( = −108.6 kJ / mol) is passed into water ( = −241.5 kJ / mol), the hydrate is formed ( calc . = −380.9 kJ / mol). This hydration is slightly exothermic; the equilibrium is almost entirely on the side of the aldehyde hydrate .
Enthalpy of reaction dehydrogenation of methylcyclohexane
Heating methylcyclohexane ( calc . = −149.2 kJ / mol) with hydrogenation / dehydrogenation catalysts results in toluene (calc . = +50.4 kJ / mol) with elimination of hydrogen . The reaction is strongly endothermic (calc. +200 kJ / mol).
The reverse of this reaction, the hydrogenation of aromatic hydrocarbons, releases large amounts of heat. This is important when testing dibenzyltoluene for its suitability as a liquid organic hydrogen carrier .
Enthalpy of reaction hydrolysis of ethylene oxide
When adding water ( = −241.5 kJ / mol) to ethylene oxide (calc. = −52.6 kJ / mol) at 200 ° C or with acid catalysis in water, the formation of a. Ethylene glycol (calc. = −384.5 kJ / mol). As with the homologous propylene oxide, the reaction is strongly exothermic (calc. −90 kJ / mol).
The ring-opening reactions with amines (e.g. "epoxy-amine additions" in 2K adhesives) are comparable exothermic.
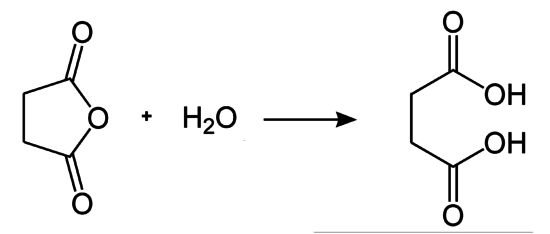
Enthalpy of reaction hydrolysis of succinic anhydride
The addition of water ( = −241.5 kJ / mol) to succinic anhydride ( calc . = −527.9 kJ / mol) forms succinic acid ( calc . = −826.8 kJ / mol). The reaction is exothermic (calc. −57 kJ / mol). For the hydrolysis of maleic anhydride , −53 kJ / mol is calculated as a comparable heat emission as for acetic anhydride or phthalic anhydride .
Carbonation of ethylene oxide and hydrolysis of ethylene carbonate ( OMEGA process )
The addition of carbon dioxide ( = −393.5 kJ / mol) to ethylene oxide (calc. = −52.6 kJ / mol) forms ethylene carbonate ( = −508.5 kJ / mol).
This partial step is exothermic at −62 kJ / mol.
In the second step, water ( = −241.5 kJ / mol) is added to this and ethylene glycol is formed ( = −384.5 kJ / mol) with the elimination of carbon dioxide .
The second sub-step is much less exothermic at −28 kJ / mol.
Further developments
The Benson method was subsequently further developed by several physical chemists against the background of powerful computer systems.
- In 1993 Domalski succeeded in predicting the enthalpies of formation of substances in the liquid or solid phase. When calculating the entropy in the liquid or solid phase, conformers are not taken into account.
- In 1996, Cohen published values for calculating the enthalpy in all three phases, which in some cases exceed the accuracy of Domalski's values.
- From 2006, Salmon and Dalmazzone published group papers on the enthalpy of formation of solids.
Web links
- NIST literature review on "Enthalpy of Education" . - N. Cohen (1996) received the order from NIST to validate and optimize the increments and to name reference values.
Individual evidence
- ↑ Sidney William Benson (September 26, 1918 - December 30, 2011)
- ↑ Sidney W. Benson and Jerry H. Buss: Additivity Rules for the Estimation of Molecular Properties. Thermodynamic Properties . In: J. Chem. Phys. . 29 , pp. 546-572 (1958). doi : 10.1063 / 1.1744539 .
- ↑ a b B. E. Poling, JM Prausnitz, JPO Connell; " The Properties of Gases and Liquids ", Fifth Edition, Mc-Graw-Hill International Editions
- ^ TH Lowry and K. Schueller Richardson, Mechanism and Theory in Organic Chemistry , Verlag Harper & Row, 1st ed. 1976, there page 71 ff (Thermochemistry).
- ↑ ES Domalski, ED Hearing ; Estimation of the Thermodynamic Properties of CHNOSX Compounds at 298K . In: J Phys Chem Ref Data , Vol. 22 , 805-1159 (1993), Example Methanol page 909.
- ↑ Hess's heat law for the calculation of heats of reaction from enthalpies of formation.
- ↑ STANDARD THERMODYNAMIC PROPERTIES OF CHEMICAL SUBSTANCES ( Memento from April 26, 2015 in the Internet Archive ), Chapter 5.4 from CRC Handbook 90th edition (2009–2010).
- ↑ Enthalpies of formation methanol and formaldehyde see Domalski 1993, pages 935 and 909, Ber. from increments and exp. Values.
- ↑ Enthalpies of formation methylcyclohexane and toluene see Domalski 1993, pages 863 and 896, Ber. from increments and exp. Values.
- ↑ Enthalpies of formation ethylene oxide and ethylene glycol see Domalski 1993, pages 932 and 917, Ber. from increments and exp. Values.
- ↑ Enthalpies of formation succinic anhydride and succinic acid see Domalski 1993, pages 964 and 951, Ber. from increments and exp. Values.
- ↑ ES Domalski, ED Hearing ; "Estimation of the Thermodynamic Properties of CHNOSX Compounds at 298K", J. Phys. Chem. Ref. Data, Vol. 22 , 805-1159 (1993)
- ^ N. Cohen ; "Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of CH and CHO Compounds", J. Phys. Chem. Ref. Data, Vol. 25 , 1411-1481 (1996)
- ↑ ; "Prediction of Enthalpy of Formation in the Solid Phase (at 298 K) using Second-Order Group Contributions - Part I: CH and CHO Compounds", J. Phys. Chem. Ref. Data, Vol. 35 , 1443-1457 (2006). - A. Salmon, D. Dalmazzone ( Memento of the original from March 29, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. ; "Prediction of Enthalpy of Formation in the Solid State (at 298 K) Using Second-Order Group Contributions - Part II: CH, CHO, and CHNO Compounds", J. Phys. Chem. Ref. Data, Vol. 36 , 19-58 (2007)