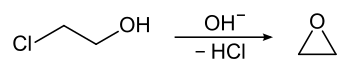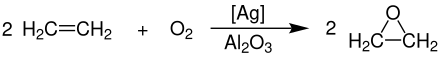Ethylene oxide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Wedges to clarify the geometry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethylene oxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 4 O | |||||||||||||||
| Brief description |
colorless gas with a sweet, ethereal odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 44.05 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
1.965 kg m −3 (0 ° C, 1013 hPa) |
|||||||||||||||
| Melting point |
−112.55 ° C |
|||||||||||||||
| boiling point |
10.5 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
easily soluble in water |
|||||||||||||||
| Refractive index |
1.3597 (7 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−78.0 kJ / mol (l) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Ethylene oxide ( EO for short ) is a colorless, extremely flammable gas with a sweet smell and the simplest epoxy . It is an important intermediate in the manufacture of ethylene glycol and other chemicals. Ethylene oxide is used as a disinfectant for food, organic insulating materials (wool, plant fibers), textile fibers and medical devices.
As mutagenic clastogen ethylene oxide is a toxic , which chromosome aberrations can cause. It is assigned the UN number 1040.
According to the IUPAC nomenclature , ethylene oxide is referred to as oxirane , the name of which is derived from the Hantzsch-Widman system .
history
Ethylene oxide was first produced in 1859 by Charles Adolphe Wurtz , for which he treated 2-chloroethanol with a base .
During the First World War, ethylene oxide gained industrial importance as the starting product for the coolant ethylene glycol. Since the chemical weapon mustard gas ( mustard ) can be produced with ethylene oxide , it falls under the Foreign Trade Act .
In 1931, the French chemist Theodore Lefort discovered the production of ethylene oxide directly from ethene and oxygen with silver as a catalyst .
Extraction and presentation
Historical procedure
Ethylene oxide was first produced in 1925 by Union Carbide Chemicals using the chlorohydrin process. For this purpose, ethylene was first reacted with chlorine in an alkaline aqueous solution to form ethylene chlorohydrin , which then reacted with calcium hydroxide to form ethylene oxide. Disadvantages of the process were a considerable waste water pollution with chlorides and the formation of halogenated hydrocarbons (e.g. 1,2-dichloroethane ) as by-products. The first catalytic direct oxidation of ethylene to ethylene oxide was also introduced technically by Union Carbide in the 1930s.
Industrial synthesis
The large-scale production of ethylene oxide now takes place exclusively through the catalytic oxidation of ethene with oxygen at temperatures of 230-270 ° C and pressures of 10-20 bar. The catalyst used is finely divided silver powder which is applied to an inorganic , oxide-containing carrier (preferably aluminum oxide ).
The complete reaction is carried out in a tube bundle reactor in which the considerable heat of reaction (ΔH R = –119.7 kJ · mol −1 of the main and ΔH R = –1324 kJ · mol −1 of the side reaction) is dissipated with the help of molten salts Generation of superheated high pressure steam is used. In this process, the catalyst is arranged as a fixed bed . The yield of pure ethylene oxide is 85%. The complete oxidation of the ethene to carbon dioxide and water occurs as a side reaction .
In 2010 around 21 million tons of ethylene oxide were used worldwide.
properties
Ethylene oxide is an extremely flammable gas. The flash point is −57 ° C and the ignition temperature is 435 ° C. It forms explosive mixtures with air, the lower explosion limit (LEL) is 2.6%, the upper (UEG) is 100%.
use
Ethylene oxide gas kills bacteria, viruses and fungi, so it can be used to fumigate heat-sensitive substances. The sterilization of spices with EO was patented in 1938 by the American chemist Lloyd Hall and is still practiced in some countries today. In Germany, the use of ethylene oxide in the food sector has been banned since 1981, as this can produce toxic 2-chloroethanol . Sterilization with ethylene oxide is now a widespread process in the industrial manufacture of medical products, in particular single-use products such as bandages, sutures or syringes and catheters, but also surgical instruments and sensitive medical products (e.g. cochlear implants ). The process is highly standardized (including ISO 11135, ISO 10993-7, EN 1422). Treating cotton swabs with ethylene oxide gas can degrade traces of DNA to such an extent that they can no longer be detected using forensic methods .
Most ethylene oxide is used as an intermediate in the manufacture of other chemicals. Much of the ethylene oxide is used in the production of ethylene glycol , now through the OMEGA process . It is also required for the production of polyesters (e.g. PET ) or hydroxyethyl cellulose (HEC). Only about 2% of world production is used for sterilization with gaseous EO.
Ethylene oxide can polymerize to form polyethylene glycol (also known as polyethylene oxide), which is a non-toxic and readily water-soluble polymer. It is also important for the manufacture of surfactants (see Nonionic Surfactants ), e.g. B. polyalkylene glycol ethers .
One category of ethylene oxide derivatives that has received a great deal of scientific attention are the crown ethers , which are cyclic oligomers of ethylene oxide. These compounds have the ability to make ionic substances soluble in non-polar solvents in which they are otherwise insoluble. Due to the high costs, the use of these substances remains limited to laboratory applications.
In the military sector, ethylene oxide is used as a fuel in small aerosol bombs that are used e.g. B. in cluster bombs of the type CBU-55 are used.
Use in stored product protection
Ethylene oxide was - in order to reduce the flammability - together with a higher proportion of carbon dioxide under the trade names Cartox and T-Gas as a fumigant from z. B. silos, storage rooms and containers are used.
safety instructions
Ethylene oxide is toxic and carcinogenic if inhaled. Symptoms of poisoning include headache, dizziness, and nausea / vomiting. With increasing dose it comes to twitching, cramps and finally to a coma. It is irritating to the skin and the respiratory tract. The lungs may fill with fluid hours after you inhale ( pulmonary edema ).
Ethylene oxide is normally stored under pressure in combination with 10% carbon dioxide . At normal pressure and room temperature, it evaporates very quickly and causes frost burns on the skin.
In animals it has caused numerous reproductive defects such as mutations or miscarriages. The influence on human reproduction has not yet been studied in detail, but it is likely that the same effects will occur as in animal experiments.
In 2012, ethylene oxide was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reason for the uptake of ethylene oxide were concerns about its classification as a CMR substance, high (aggregated) tonnage. The reassessment took place from 2012 and was carried out by Austria . A final report was then published.
See also
- Ethylenimine (aziridine), the nitrogen-containing analog
- Thiirane (ethylene sulfide), the sulfur-containing analogue
literature
- Wolfgang Swodenk, Helmut Waldmann: Modern processes in large-scale chemistry: ethylene oxide and propylene oxide. In: Chemistry in Our Time . Vol. 12, No. 3, 1978, ISSN 0009-2851 , pp. 65-70
Individual evidence
- ↑ a b c d e f g h i j k l m n Entry on ethylene oxide in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-408.
- ↑ Entry on Ethylene oxide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 75-21-8 or ethylene oxide ), accessed on September 13, 2019.
- ↑ CRC Handbook 90th edition (2009–2010), pp. 5–22 ( Memento of April 26, 2015 in the Internet Archive ). - see also entry on ethylene oxide . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed on 22 March 2010 .
- ↑ Foreign Trade Act ( Memento of the original dated June 6, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 1.3 MB) in the version of the announcement of May 27, 2009 ( Federal Law Gazette I p. 1150 ), which has been changed by Article 1 of the Ordinance of December 12, 2012 (BAnz. 2012). P. 200.
- ↑ a b c Manfred Baerns, Arno Behr, Axel Brehm, Jürgen Gmehling, Kai-Olaf Hinrichsen, Hanns Hofmann, Regina Palkovits, Ulfert Onken, Albert Renken: Technische Chemie . 2nd Edition. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany 2013, ISBN 978-3-527-33072-0 , p. 16 f., 592 .
- ↑ Max Daunderer: Handbook of environmental toxins. Hüthig Jehle Rehm Publishing Group, 6/2006 edition.
- ↑ Jens Lubbadeh: Forensic DNA analysis - weak point cotton swab , message from March 26, 2009 in Spiegel Online Wissenschaft.
- ↑ BAK to BSU / BSG - Equipment Listing Designation-Systems.net, accessed May 30, 2013.
- ^ European Chemicals Agency (ECHA): Substance Evaluation Report and Conclusion Document .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Ethylene oxide , accessed on March 26, 2019.