Side reaction
A side reaction is a chemical reaction that occurs at the same time as the main reaction itself, but to a lesser extent. It leads to the formation of by- product , which reduces the yield of the main product:
Here P 1 is the main product if k 1 > k 2 . The by-product P 2 is also generally undesirable and has to be separated off from the actual main product (usually in a complex manner).
In organic synthesis
B and C from the equation above usually represent different compounds. However, B and C can also be different positions in a molecule. Connections that can react in different positions are called ambifunctional or ambident .
A side reaction is also referred to as a competitive reaction when different compounds (B, C) compete for another reactant (A). If the secondary reaction takes place as frequently as the main reaction, it is referred to as parallel reactions (especially in the kinetics, see below ).
There can also be more complicated relationships. Compound A could react reversibly but quickly to substance B (with speed k 1 ) or irreversibly but slowly (k 1 > k -1 >> k 2 ) to substance C:
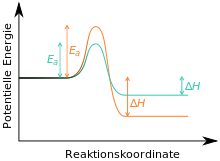
The reaction to substance C could be irreversible because it is thermodynamically very stable. In this case, B would be the kinetic and C the thermodynamic product of the reaction (see also here ). If the reaction is carried out at low temperatures and terminated after a short time, it is called kinetic control ; the kinetic product B would have formed primarily. If the reaction is carried out at high temperature and for a long time (then the necessary activation energy for the reaction to form C is available), which is increasingly formed over time, one speaks of thermodynamic control ; the thermodynamic product C is formed.
Conditions for side reactions
In organic synthesis, an increased temperature usually leads to more by-products. Since these are generally undesirable, low temperatures (“mild conditions”) are chosen. The relationship between competing reactions can usually be influenced by changing the temperature, since their activation energies are usually different. Reactions with a high activation energy are accelerated more by increasing the temperature than those with a low activation energy. The position of equilibrium is also temperature dependent.
Detection reactions can be falsified by side reactions.
kinetics
Side reactions are also described in reaction kinetics , a branch of physical chemistry . Side reactions are understood as complex reactions, since the overall reaction (main reaction + side reaction) is composed of several (at least two) elementary reactions. Other complex reactions are competing reactions, parallel reactions, subsequent reactions, chain reactions, reversible reactions, etc.
If one reaction takes place significantly faster than the other (k 1 > k 2 ), this (k 1 ) is called the main reaction and the other (k 2 ) is called a side reaction. If both reactions take place at about the same speed (k 1 ≅ k 2 ) we speak of parallel reactions .
If the reactions and proceed irreversibly (without reverse reaction), then the ratio of P 1 and P 2 corresponds to the relative reactivity of B and C to A:
Individual evidence
- ↑ side reaction on merriam-webster.com . Retrieved August 30, 2015.
- ↑ Competitive reaction to chemgapedia.de . Retrieved August 30, 2015.
- ↑ Competitive reaction to universal_lexikon.deacademic.com . Retrieved August 30, 2015.
- ↑ a b 4. Kinetics and Catalysis (PDF) Accessed August 30, 2015.
- ↑ Kinetic and thermodynamic control of chemical reactions on Chemgapedia.de . Retrieved December 6, 2015.
- ↑ John Gilbert, Stephen Martin: Experimental Organic Chemistry: A Miniscale and Microscale Approach . 2010, ISBN 978-1-4390-4914-3 , pp. 445 ( limited preview in Google Book search).
- ^ Robert G. Mortimer: Physical Chemistry . Academic Press, 2008, ISBN 978-0-08-087859-1 ( limited preview in Google Book Search).
- ^ Klaus Schwetlick: Organikum . 23rd edition. Wiley-VCH, Weinheim 2009, ISBN 978-3-527-32292-3 , pp. 156 .
- ↑ Complex reactions on Spektrum.de . Retrieved August 30, 2015.
- ↑ Claus Czeslik, Heiko Seemann, Roland Winter: Basic knowledge of physical chemistry . Vieweg + Teubner, 2010, ISBN 978-3-8348-9359-8 , pp. 280–291 ( limited preview in Google Book search).



![{\ displaystyle \ mathrm {{\ frac {[P_ {1}]} {[P_ {2}]}} = {\ frac {k_ {1} [B]} {k_ {2} [C]}}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/5c7d372c2484615803867ad7fdfed219d096866c)