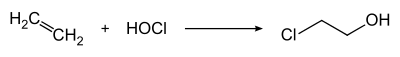2-chloroethanol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
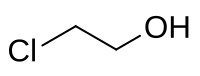
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-chloroethanol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 5 ClO | |||||||||||||||
| Brief description |
colorless liquid with an ethereal odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 80.51 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.21 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−70 ° C |
|||||||||||||||
| boiling point |
129 ° C (1013 hPa) |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
miscible with water |
|||||||||||||||
| Refractive index |
1.4419 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−295.4 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-chloroethanol , often referred to as ethylene chlorohydrin denotes a is chloro - derivative of the ethanol is one that the most toxic organic halogen compounds.
Occurrence
2-chloroethanol can arise in foods , especially in spices that have been sterilized with ethylene oxide . In Germany, fumigation with ethylene oxide was permitted until 1981 in order to kill viruses, bacteria and fungi. Since then, this type of sterilization has been banned in the food sector because it is now known that both ethylene oxide and its conversion product, 2-chloroethanol, are highly toxic and mutagenic. However, in many third countries fumigation with ethylene oxide, for example before shipping by ship, is still the method of choice.
Manufacturing
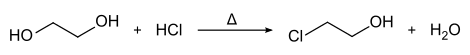
In the laboratory, 2-chloroethanol can be produced from ethylene glycol by heating it with hydrogen chloride .
Technically, it is produced by chlorohydration of ethene with hypochlorous acid (HOCl).
The HOCl can be formed from chlorinated lime with chlorine gas in the aqueous phase or directly by introducing chlorine into water under pressure.
properties
Physical Properties
The colorless liquid has a weak, pleasantly sweet odor reminiscent of ether . 2-chloroethanol boils at 129 ° C. under normal pressure . The heat of vaporization at the boiling point is 45.7 kJ mol −1 . According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 5.44166, B = 2082.063 and C = −18.844 in the temperature range from 269 to 402 K. The compound is miscible with many alcohols and water.
Safety-related parameters
2-chloroethanol forms flammable vapor-air mixtures above the flash point temperature. The compound has a flash point of 55 ° C. The explosion range is between 5.0% by volume (160 g / m 3 ) as the lower explosion limit (LEL) and 16.0% by volume (540 g / m 3 ) as the upper explosion limit (UEL). The ignition temperature is 425 ° C. The substance therefore falls into temperature class T2.
use
2-chloroethanol has a wide range of uses in the synthesis of dyes , insecticides , anesthetics and plasticizers . Mainly as a reagent for hydroxyethylation .
It is primarily used for the production of ethylene oxide . Occasionally it is used as a solvent for cellulose acetate and ethyl cellulose . The trade and transport of 2-chloroethanol has only a very small volume. Usually it is generated directly on site and processed further.
toxicology
2-chloroethanol is a dangerous poison because after skin contact with the liquid there is usually no local irritant effect that could serve as a warning sign. Any contact with the vapors or the liquid must therefore be avoided at all costs. Skin absorption has resulted in multiple deaths. 2-chloroethanol vapors irritate the eyes and the respiratory tract. The central nervous system becomes paralyzed and liver and kidney damage occurs. Incineration produces, among other things, hydrogen chloride and the highly toxic phosgene .
Even if a carcinogenic effect in animal experiments is reported in older sources , more recent studies show that this is not the case. In the tests, however, the substance shows a moderately mutagenic potential.
literature
- Wallace L.Guess: Tissue reactions to 2-chloroethanol in rabbits . In: Toxicology and Applied Pharmacology . tape 16 , no. 2 , 1970, p. 382-390 , doi : 10.1016 / 0041-008X (70) 90009-8 .
- JV Bruckner, Wallace L. Guess: Morphological skin reactions to 2-chloroethanol . In: Toxicology and Applied Pharmacology . tape 22 , no. 1 , 1983, p. 29-44 , doi : 10.1016 / 0041-008X (72) 90222-0 .
Individual evidence
- ↑ a b c d e f g h i j k l m n o Entry on 2-chloroethanol in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-100.
- ↑ Entry on 2-chloroethanol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet 2-chloroethanol from Sigma-Aldrich , accessed on January 25, 2020 ( PDF ).
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for 107-07-3 or 2-chloroethanol ), accessed on November 2, 2015.
- ↑ University of Würzburg: Operating Instructions 2-Chlorethanol , accessed on July 6, 2007 ( Memento from September 30, 2007 in the Internet Archive )
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-22.
- ↑ a b c d e Entry on ethylene chlorohydrin. In: Römpp Online . Georg Thieme Verlag, accessed on August 8, 2016.
- ↑ Max Daunderer: Handbook of environmental toxins. Hüthig Jehle Rehm Publishing Group, 6/2006 edition
- ↑ Spices - Hot and Poisonous. ( Memento from September 30, 2007 in the Internet Archive ) ÖKO-TEST November 95, accessed on July 6, 2007.
- ↑ J. Fowles, J. Mitchell, H. McGrath: Assessment of cancer risk from ethylene oxide residues in spices imported into New Zealand . In: Food and Chemical Toxicology . tape 39 , no. 11 , 2001, p. 1055-1062 , doi : 10.1016 / S0278-6915 (01) 00052-7 .
- ↑ L. Smith, S. Skyle: "The mode of formation of the chlorohydrins. VIII. On the addition of hypochlorous acid to a double bond.", In Acta Chem. Scand. 1950 , 4 , pp. 39-44. doi: 10.3891 / acta.chem.scand.04-0039
- ^ A b Stull, DR: Vapor Pressure of Pure Substances. Organic and Inorganic Compounds in Ind. Eng. Chem. 39 (1947) 517-540, doi: 10.1021 / ie50448a022 .
- ^ A b c E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ C. Malaveille, H. Bartsch, A. Barbin, AM Camus, R. Montesano, A. Croisy, P. Jacquignon: Mutagenicity of vinyl chloride, chloroethyleneoxide, chloroacetaldehyde and chloroethanol . In: Biochemical and Biophysical Research Communications . tape 63 , no. 2 , 1975, p. 363-370 , doi : 10.1016 / 0006-291X (75) 90697-X .
- ↑ Ada GAC Knaap, CE Voogd, PGN Kramers: Comparison of the mutagenic potency of 2-chloroethanol, 2-bromoethanol, 1,2-epoxybutane, epichlorohydrin and glycidaldehyde in Klebsiella pneumoniae, Drosophila melanogaster and L5178Y mouse lymphoma cells . In: Mutation Research / Genetic Toxicology . tape 101 , no. 3 , 1982, pp. 199-208 , doi : 10.1016 / 0165-1218 (82) 90153-7 .
- ↑ Hiroki Sakai, Tetsuya Tsukamoto, Masami Yamamoto, Kiyoshi Kobayashi, Hirofumi Yuasa, Toshio Imai, Tokuma Yanai, Toshiaki Masegi, Masae Tatematsu: Distinction of carcinogens from mutagens by induction of liver cell foci in a model for detection of initiation activity . In: Cancer Letters . tape 188 , no. 1-2 , 2002, pp. 33-38 , doi : 10.1016 / S0304-3835 (02) 00009-5 .