Chlorohydration
Chlorohydration is the addition of hypochlorous acid to an olefinic double bond. This creates chlorine alcohols, so-called chlorohydrins , in which the chlorine and the hydroxyl group are bound to neighboring carbon atoms.
use
Chlorohydrins may be prepared by elimination of hydrogen chloride to epoxides implement.
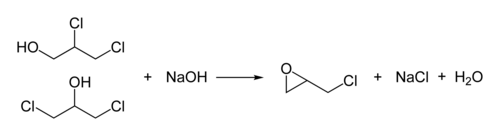
The production of epichlorohydrin is of technical importance . By chlorohydration of allyl chloride with hypochlorous acid, 1,3-dichloropropan-2-ol and 2,3-dichloropropan-1-ol are obtained:
Reaction with sodium hydroxide produces racemic epichlorohydrin:
Propylene chlorohydrin (PCH) and propylene oxide can be obtained in the same way by chlorohydrination of propene .
literature
- E. Bartholomé (Ed.), E. Biekert (Ed.), H. Hellmann (Ed.): Ullmanns Encyklopädie der technischen Chemie . Wiley-VCH, 1984, ISBN 978-3527200009 .
Individual evidence
- ↑ E. Buss, A. Rockstuhl, FRD Schnurpfeil: Investigations on the mechanism of the chlorohydrination of olefins. In: Journal for Practical Chemistry. 324, 1982, pp. 197-208, doi : 10.1002 / prac.19823240204 .
- ↑ Patent DE 19614683 , process for the production of propylene oxide by chlorohydration and alkali lye saponification , filed April 13, 1996.