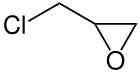
Epichlorohydrin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| Simplified structural formula without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Epichlorohydrin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 3 H 5 ClO | ||||||||||||||||||
| Brief description |
colorless, pungent smelling liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 92.53 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.18 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−48 ° C |
||||||||||||||||||
| boiling point |
116 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.4358 (25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
Switzerland: 2 ml m −3 or 8 mg m −3 |
||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−148.4 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Epichlorohydrin is a colorless , low- viscosity liquid that smells of chloroform . It was recognized in animal experiments as clearly carcinogenic, which is why no MAK value can be given. Most epichlorohydrin is synthesized as a racemate . Whenever epichlorohydrin is mentioned without further name components, the racemate [1: 1 mixture of ( R ) -epichlorohydrin and ( S ) -epichlorohydrin] is always meant. The pure enantiomers are also known, but are of little importance.
Extraction and presentation
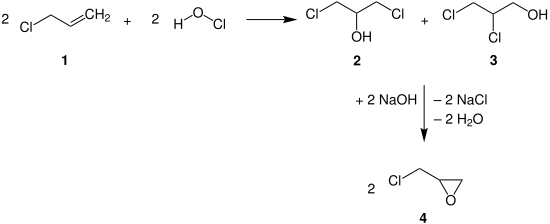
The conventional production of epichlorohydrin 4 is performed by the reaction of allyl chloride 1 with hypochlorous acid to 1,3-dichloropropane-2-ol 2 and 2,3-dichloropropane-1-ol 3 and subsequent treatment with sodium hydroxide (NaOH) to epichlorohydrin 4 .
Another production possibility is based on the use of glycerine (e.g. from biodiesel production ), which is also converted into 1,3-dichloropropan-2-ol via hydrochloric acid and then converted into epichlorohydrin with NaOH . In these reactions, epichlorohydrin is obtained as a racemate .
properties
Chemical properties
Epichlorohydrin reacts quickly with nucleophilic compounds, e.g. B. amines , with opening of the epoxy ring. Under suitable reaction conditions, it reacts by splitting off chloride and forming a new epoxide . It is therefore used by industry to introduce the epoxy group into, for example, resins .
Safety-related parameters
Epichlorohydrin forms highly flammable vapor-air mixtures. The compound has a flash point of 28 ° C. The explosion range is between 2.3 vol.% (86 g / m 3 ) as the lower explosion limit (LEL) and 34.4 vol.% (1315 g / m 3 ) as the upper explosion limit (UEL). The lower explosion point is 26 ° C. The limit gap width was determined to be 0.74 mm. This results in an assignment to explosion group IIB. The ignition temperature is 385 ° C. The substance therefore falls into temperature class T2.
use

Until a few years ago, a large proportion of epichlorohydrin was used in the production of glycerine and glycidol , but the proportion of synthetic glycerine production fell sharply due to the large amounts of glycerine that result from biodiesel production.

Above all as a component in the production of epoxy resins , which, in addition to their use as materials, are also used as wet strength agents in the production of paper (e.g. in the production of tea bag paper and kitchen paper ) and as two-component adhesives ( bisphenol A resins) Epichlorohydrin plays a big role. It is also part of synthetic elastomers and textile auxiliaries such as the polyamide- epichlorohydrin polymer Hercosett , which is used as a shrinkage inhibitor in textiles.
safety instructions
Epichlorohydrin is toxic and carcinogenic. It can diffuse through intact skin .
proof
The substance can be detected using test tubes , gas chromatography or a PID sensor .
literature
- Ullmann 5th edition Volume A9 p. 539 f; Beilstein EIII 17.
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q Entry on 1-chloro-2,3-epoxypropane in the GESTIS substance database of the IFA , accessed on October 11, 2019(JavaScript required) .
- ↑ Entry on α-epichlorohydrin. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-228.
- ↑ Entry on 1-chloro-2,3-epoxypropane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for 106-89-8 or 1-chloro-2,3-epoxypropane (epichlorohydrin) ), accessed on November 2, 2015.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-23.
- ↑ Bruce M. Bell et al .: Glycerin as a Renewable Feedstock for Epichlorohydrin Production. The GTE Process. ( Memento of December 30, 2009 in the Internet Archive ) (PDF; 697 kB) Clean 36 (8), 2008; Pp. 657-661.
- ↑ a b c d e E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.