1-chloro-2-propanol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 1-chloro-2-propanol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 7 ClO | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 94.54 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.10 g cm −3 |
|||||||||||||||
| boiling point |
127 ° C |
|||||||||||||||
| Vapor pressure |
6.5 mbar (20 ° C) |
|||||||||||||||
| solubility |
good in water (> 100 g l −1 at 23 ° C) |
|||||||||||||||
| Refractive index |
1.4392 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data |
720 mg · kg -1 ( LD 50 , guinea pigs , oral ) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1-chloro-2-propanol is a chemical compound from the group of alcohols .
Extraction and presentation
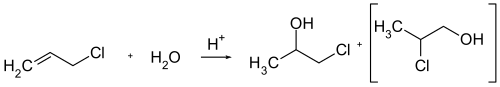
1-Chloro-2-propanol can be obtained by the acid-catalyzed reaction of allyl chloride with water or the chlorohydration of propylene (which also produces the isomeric 2-chloro-1-propanol ).
The compound is also formed as a by-product of the thermal decomposition of tris (2-chloroisopropyl) phosphate .
properties
1-chloro-2-propanol is a flammable, not very volatile, colorless liquid that is miscible with water.
Stereoisomerism
1-chloro-2-propanol is chiral , i.e. it contains a stereocenter . There are thus two stereoisomers, the ( R ) enantiomer and the ( S ) enantiomer. The 1: 1 mixture ( racemate ) of ( R ) - and ( S ) -enantiomer is called ( RS ) -1-chloro-2-propanol or (±) -1-chloro-2-propanol. When “1-chloro-2-propanol” is mentioned in the scientific literature or in this article without further details, the racemate is meant.
| Enantiomers of 1-chloro-2-propanol | ||||
| Surname | ( R ) -1-chloro-2-propanol | ( S ) -1-chloro-2-propanol | ||
| Structural formula |

|
|||
| CAS number | 19141-39-0 | 37493-16-6 | ||
| EC number | 686-912-5 | 684-565-4 | ||
| ECHA info card | 100.212.853 | 100.210.009 | ||
| PubChem | 6999046 | 641044 | ||
use
1-Chloro-2-propanol is used as an intermediate in the production of other chemical compounds such as propylene oxide or 3-hydroxybutanoic acid .
Individual evidence
- ↑ a b c d e f g h Entry on 1-chloro-2-propanol in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c Entry on 1-chloro-2-propanol in the Hazardous Substances Data Bank , accessed on September 16, 2012.
- ↑ Data sheet 1-chloro-2-propanol, 70% from Sigma-Aldrich , accessed on June 25, 2016 ( PDF ).
- ↑ Martin Klein: A contribution to the recording of emissions during the thermal load of rigid polyurethane foams . Herbert Utz Verlag, 1999, ISBN 978-3-89675-658-9 , p. 133 ( limited preview in Google Book search).