Chirality (chemistry)
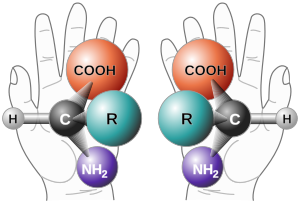
In stereochemistry, chirality describes a spatial arrangement of atoms in a molecule in which simple symmetry operations , e.g. B. a reflection on a molecular plane, does not lead to a self- image. (Expressed geometrically precisely, this means: a point reflection - identical to a rotational reflection - is not possible, but an axis reflection .) Here, single or multiple atoms in a molecule can represent one or more stereogenic centers and the entire molecular shape can account for the chirality. Molecules with this property are called chiral , molecules without this property are called achiral . Chirality is a Greek made-up word and means "handedness", derived from the root word χειρ ~, ch [e] ir ~ - hand ~ . In crystallography it is also called enantiomorphism .
Common examples from everyday life, right and left hand , right hand or left-handed screw houses or screws , as well as "normal" corkscrew with right-hand thread (for right-handed ), and corkscrew with left-hand thread (for left-handed ). Even dice are chiral, in mirror-image arrangement of the numbers the forms can not be made to coincide (see figure).
In general, an object is chiral if and only if it does not have a rotating mirror axis . However, other symmetry elements can certainly be present, i. H. a chiral object is not necessarily asymmetric .
Chemistry in general
Chirality is mostly based on the different spatial arrangement of atoms and groups of atoms around one or more stereocenters. So make z. B. carbon atoms with four different substituents represent a stereogenic center or stereocenter in which two different spatial arrangements are possible. In addition to carbon, other atoms such as phosphorus can also form stereocenters. The decisive factor here is that the substituents cannot change their position relative to one another, which in the case of phosphorus is ensured by a sufficiently large inversion barrier. Nitrogen can usually only function as a stereocenter in strained systems, since nitrogen would otherwise oscillate at a high frequency and thus constantly invert it. The lone pair of electrons is the fourth substituent on the chiral nitrogen center . This has the lowest priority of all substituents.

| Everyday item: corkscrews are chiral |
| Commercial ("normal") corkscrew for right-handers. |
| Left-handed corkscrew. |
Molecules whose image and mirror image cannot be matched are therefore chiral. The two thus distinguishable mirror-image forms of such a molecule are called enantiomers . The enantiomers can be distinguished by their different optical activity . A mixture with equal proportions of both enantiomers is called a racemate or racemic mixture.
In the simplest case in organic chemistry there is chirality when a carbon atom in a molecule bears four different substituents. This carbon atom is called the stereocenter (sometimes obsolete as the chiral center or asymmetric carbon atom). Fundamental considerations and measurements of the chirality of correspondingly substituted carbon compounds go back to Jacobus Henricus van 't Hoff and (later) Paul Walden . The spatial arrangement of the substituents at a stereocenter is designated by ( R ) or ( S ) according to the rules ( CIP rules ) established by RS Cahn , CK Ingold and V. Prelog . ( R ) stands for rectus, Latin for “right”, and ( S ) for sinister, Latin for “left”.
If there are several stereocenters, the number of possible different connections also increases. With n stereocenters, there are 2 n different compounds, minus possible meso compounds (see below). The stereocenters are then individually designated with ( R ) or ( S ) according to the CIP rules . If two compounds differ in one or more, but not in all stereocenters, one speaks of diastereomers . If a molecule has several stereocenters, but these can be converted into one another through a reflection at one plane, the entire molecule is achiral. In this case, one speaks of meso compounds (e.g. meso tartaric acid ).
An older convention for naming enantiomers, which is still used today for sugars and partly also for amino acids , is the D and L nomenclature of the Nobel Prize winner Emil Fischer ( Fischer projection ).
In addition to this chirality (central chirality) attributed to stereocenters, a distinction is made between axial , planar and helical chirality in order to describe the underlying structural elements in more detail. Axial chirality occurs e.g. B. in biphenyls such as BINAP , which are substituted in the ortho positions that the free rotation of the aromatics around the CC single bond is greatly hindered. This then results in two mirror-image isomers. Examples of planar chirality are E - cyclooctene or certain sandwich complexes or substituted cyclophanes . Helical chirality is understood to mean the different directions of rotation of helical connections. Helical chirality occurs e.g. B. at Helicenen .
Common to all chiral compounds is the absence of a rotating mirror axis . As can be proven from group theory , this absence of a rotating mirror axis is the necessary and sufficient condition for a molecule to appear in enantiomers. A rotating mirror axis S n is an element of symmetry. Here you first rotate the molecule 360 / n degrees around an axis and then mirror it on the plane that is perpendicular to this axis. If the product of this operation is identical to the initial compound, a rotating mirror axis has been found.
The terms pseudochirality and prochirality also exist . Two of the substituents on a pseudochiral (= pseudo-asymmetric) center differ only in their configuration , and are therefore enantiomorphic. As a result, they lie on a mirror plane of the molecule (provided there are no other stereogenic centers), making it an achiral, so-called meso compound. (The term “pseudochirality” is sometimes also used when both substituents have the same configuration and the molecule is therefore chiral.). Compared to the R / S nomenclature ( CIP nomenclature ), pseudochiral centers are designated with r and s , with the ( R ) -configured substituent receiving higher priority. Prochiral groups are those functions that can be converted into a stereocenter by adding them to themselves. As a good example, unsymmetrical ketones serve here , which can be converted into chiral alcohols , for example by hydrogenation . A distinction is made between the attack and the re or si side.
Chirality also occurs in inorganic chemistry . As Alfred Werner ( Nobel Prize 1913) was able to show in 1911, coordination compounds with octahedral geometry can also exhibit chirality. A simple example is the complex Co (en) 3 3+ , where en stands for the bidentate ligand 1,2-diamino-ethane. The two enantiomeric forms of this complex and similar structures are usually denoted by Δ and Λ. Coordination compounds show a very large variety of possible chiral structures. At the beginning of the 21st century, chiral coordination compounds became very important, especially because of their applications in catalysis. Chirality can also occur in inorganic solids . For example, quartz has two enantiomorphic forms that can be traced back to left-handed or right-handed screws. This is also an example of helical chirality. In crystallography there are a total of eleven enantiomorphic point groups .
The absolute configuration of a chiral substance cannot be inferred from the sense of rotation of polarized light when passing through a standard solution, but must either be drawn by chemical analogy (for example, by breaking down the substance to be determined into a known compound), by X-ray crystallography or by using chiral shift reagents in the NMR - spectroscopy carried out. Only after such evidence can a decision be made as to whether a compound has an ( R ) or ( S ) configuration. The assignment of the configurations for amino acids and carbohydrates, of which only the relative configurations were initially known, was initially arbitrary. Much later (in the 1950s), X-ray crystallographic examinations showed that the selected assignment coincidentally (note: probability was 50%) corresponds to the actual circumstances.
The investigation of the chirality of molecules in the gas phase can be carried out using the circular dichroism effect with synchrotron radiation . A new research approach now uses circularly polarized light from a femtosecond laser . Since January 2018, the German Research Foundation has been funding a special research area for research into the chirality of molecules, which is coordinated by scientists from the University of Kassel .
biochemistry
The concept of chirality also plays a fundamental role in biology , especially in biochemistry . In all classes of natural substances, one enantiomer is preferred or present exclusively. So one finds in nature z. B. only D - glucose and no L - glucose . (There are, however, L sugar and even sugar that occur in both the D and L forms, but in completely different contexts, see monosaccharides ). Many stereoselective syntheses and enantiomerically pure synthetic compounds can be traced back to this natural reservoir of enantiomerically pure substances, the chiral pool , in direct or indirect form, for example through stereoselective catalysts .
Biochemical reactions are catalyzed by enzymes . Since enzymes are chiral macromolecules, they are able to control a reaction enantioselectively . This happens through a diastereoselective (!) Mechanism, whereby of the two enantiomeric transition states of the substrate the one whose energy is lower, that is to say which is stabilized by the active center, is preferred. This enables chiral products to be synthesized from prochiral and achiral educts . In this way, the preference for one enantiomer and with it the chirality continues throughout biochemistry and physiology . Chirality is also the prerequisite for ordered secondary structures in proteins such as B. an α- helix which can only be built up from enantiomerically pure amino acids (in nature L- amino acids). Thus, enantiomeric chiral molecules usually show different physiological effects, they have a different taste, smell, different toxicity and a different pharmacological effect as a medicinal substance. (In the most famous case Contergan ® / thalidomide the different effects of the enantiomers is, however, not specify the precise location as it in vivo to a racemization occurs and therefore the differing physiological effect of the enantiomers 'leveled' and irrelevant so).
Strictly speaking, for this reason a “racemic biology” in the sense of a 1: 1 mixture of all enantiomers would not be possible at all, since a completely separate, mirror-inverted synthesis apparatus would be necessary for the mirror-inverted molecules. The rare D- amino acids found in some bacterial cell walls in proteinogenically bound form are synthesized via a secondary metabolism and prevent z. B. the degradation by proteases .
It has not yet been clarified whether the preference found for a certain enantiomer of biomolecules is based on a random selection at the beginning of the evolutionary chain, which then strengthened itself, or whether there are fundamental reasons for preferring this configuration. Because of the parity violation already mentioned above in the weak interaction , the energy content of two enantiomers is not exactly the same. However, the difference in energy is so small that the question can rightly be asked whether it is significant at all.
Biocatalysis
In the case of biocatalytic transformations, use is made of the fact that in reactions with enzymes as the biocatalyst, an enantiomer is usually formed in excess, or if a racemate is presented as the starting material, one enantiomer is preferentially converted by the enzyme. Thus, starting from a racemic ester, the ester group of one enantiomer of the ester can be hydrolyzed under the stereoselective influence of the enzyme pancreatic lipase , while the other enantiomer of the ester remains unchanged. The enantiomerically pure carboxylic acid can then easily be separated from the also largely enantiomerically pure ester using conventional separation processes ( crystallization , chromatography, etc.). The enzyme aspartase can catalyze the enantioselective addition of ammonia (NH 3 ) to the C = C double bond of fumaric acid, resulting in ( S ) - aspartic acid .
See also
literature
- Pedro Cintas: Origins and development of the terms chirality and handedness in chemical language. Angewandte Chemie 119 (22), pp. 4090-4099 (2007), doi : 10.1002 / anie.200603714 .
- Henri Brunner : Right or Left , Wiley-VCH Verlag, Weinheim / Bergstasse, 1999, ISBN 3-527-29974-2 .
- Uwe Meierhenrich : Amino Acids and the Asymmetry of Life , Springer-Verlag, Heidelberg, Berlin 2008. ISBN 978-3-540-76885-2 .
- Anne J. Rüger, Joshua Kramer, Stefan Seifermann, Mark Busch, Thierry Muller, Stefan Bräse: handedness - life in a chiral world . In: Chemistry in Our Time . tape 46 , no. 5 , 2012, p. 294–301 , doi : 10.1002 / ciuz.201200579 .
Web links
- ChemgaPedia: Axial Chirality , Helical Chirality , Planar Chirality
Individual evidence
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 747.
- ^ A. Werner, VL King: Ber. deutsch.chem.Ges. 44 , 1887 (1911).
- ^ A. von Zelewsky: Stereochemistry of Coordination Compounds. Wiley, Chichester, 1996. ISBN 978-0471955993 .
- ↑ H. Amouri, M. Gruselle: Chirality in Transition Metal Chemistry. Wiley, Chichester, 2008. ISBN 978-0470060544 .
- ↑ Christian Lux, Matthias Wollenhaupt u. a .: Circular dichroism in the photoelectron angle distributions of camphor and fenchone from multiphoton ionization with femtosecond laser pulses. In: Angewandte Chemie. 124, 2012, pp. 5086-5090, doi : 10.1002 / anie.201109035 .
- ↑ Press release of the German Research Foundation: http://www.dfg.de/service/presse/pressemitteilungen/2017/pressemitteilung_nr_48/
- ↑ EJ Ariëns: Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology , European Journal of Clinical Pharmacology 26 (1984) 663-668, doi : 10.1007 / BF00541922 .
- ↑ Hisamichi Murakami: From Racemates to Single Enantiomers - Chiral Synthetic Drugs over the last 20 Years , Topics in Current Chemistry 269 (2007) 273-299, doi : 10.1007 / 128_2006_072 .


