Tartaric acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| D - (-) - shape (top left) and L - (+) - shape (top right) and meso shape (bottom) | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tartaric acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 6 O 6 | ||||||||||||||||||
| Brief description |
colorless and odorless solid with a sour taste |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 150.09 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
|
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| pK s value |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data |
7500 mg kg −1 ( LD Lo , rat , oral , L - (+) - tartaric acid) |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tartaric acid , also known as 2,3-dihydroxysuccinic or 2,3-dihydroxybutanedioic or tartaric acid , in Latin as Acidum tartaricum and English with tartaric acid refers to the Greek tartaros hell, because of the corrosive, burning effect.
It is a dicarboxylic acid in the α- hydroxycarboxylic acid group . It belongs to the sugar dicarboxylic acids ( aldaric acids ), their salts and esters are called tartrates . L - (+) - tartaric acid occurs, for example, in grapes and is approved as a food additive E 334 in the EU . In Germany, the total acidity of wines - calculated as tartaric acid - is given, although a number of other acids, especially malic acid , occur in wine .
Grape acid is the name of the racemate of tartaric acid. The intermolecular elimination of water produces the polymeric metatartaric acid , which is also used as a food additive under the name E 353 .
history
Before the discovery of tartaric acid, its salt, potassium hydrogen tartrate , or tartar , was thought to be an acid, since it precipitates easily in wine due to its poor water solubility and is therefore easier to identify for chemists than the readily soluble tartaric acid. At that time, the term wine spirit , which is still popular today, was common for pure alcohol . In 1732 Weinstein was listed as a solid acid by Boerhaave . In 1764, Marggraf detected an alkali metal in tartar, whereupon he decomposed it with calcium to calcium tartrate , which he did not investigate in detail. It was not until 1769 that Scheele , who is generally considered to be the discoverer of tartaric acid, decomposed calcium tartrate with sulfuric acid and called the separated crystalline acid "tartaric acid". The tartar was soon recognized as the acidic potassium salt of this acid.
In 1819 grape acid (Vosic acid) was discovered by the manufacturer Karl Kestner as a by-product of tartaric acid production. The isomerism of tartaric acid was established by Gay-Lussac in 1826 and examined in more detail by Pasteur , although only the optically active "dextrorotatory tartaric acid", i.e. L -tartaric acid and grape acid, called "racemic acid", were known. Pasteur made the sodium ammonium salt of these acids and discovered chirality in the process . Pasteur used a magnifying glass and tweezers to separate the crystals into left-handed and right-handed copies.
Pasteur brought the enantiomeric sodium ammonium tartrates separated from the racemate back into solution and examined their rotation value in the polarimeter . He found that both solutions of the salts obtained from the optically inactive grape acid were optically active and exhibited the same specific rotation as the sodium ammonium salt obtained from L -tartaric acid, but with opposite signs. Pasteur concluded from this that grape acid is not a pure substance , but an equal mixture of clockwise and counterclockwise tartaric acid, i.e. a racemate. The term racemate for a mixture of two enantiomers in equal parts is derived from the Latin name for grape acid (Acidum racemicum).
The interpretations of Pasteur's experiment are not limited to the fact that grape acid is a racemate of L - and D - tartaric acid. Pasteur recognized that the optical activity had to be a result of a property of the tartaric acid molecule itself. But it was not until 1874 that Le Bel and van 't Hoff , who subsequently became the first Nobel Prize winner in chemistry , were able to explain this independently on the basis of the molecular structure. Knowing that “four different objects can be arranged in two different ways in the corners of a tetrahedron , and that these arrangements relate to one another like an image and a mirror image that cannot be brought into congruence” they hypothesized that the four radicals bound to a carbon atom are arranged in a tetrahedral manner. Based on this, they assumed that optically active molecules contain at least one carbon atom with four different radicals, i.e. an asymmetric carbon atom. "Optically inactive organic substances therefore either do not contain an asymmetric carbon atom or they are mixtures of equal parts of two enantiomers."
Van 't Hoff and Le Bel recognized the tetrahedral geometry of the carbon atom on the one hand and gave a conclusive explanation for the optical activity of organic substances on the other. Only the meso -compounds could not be covered by their definition; the structure of meso -tartaric acid was only discovered much later . For a long time it was also unknown which enantiomer of tartaric acid now turns the polarization plane of light to the right and which one turns it to the left. It was not until 1951 that Bijvoet was able to use a special X-ray method based on sodium rubidium tartrate to clarify that L -tartaric acid is the dextrorotatory (+) enantiomer and D -tartaric acid is the levorotatory (-) enantiomer. By converting tartaric acid into other chemical compounds, this could also be clarified for many other pairs of enantiomers.
Occurrence
L - (+) - tartaric acid and its calcium, potassium and magnesium salts are particularly abundant in the vines, grapes and leaves of the vine, as well as in dandelions, sugar beets, tamarinds , unripe rowan berries and the seeds of the spindle tree , in the leaves of agaves , in black pepper , in pineapples and in many other fruits. During the winemaking process, sparingly soluble salts of tartaric acid are deposited as tartar on the bottom of wine barrels or wine bottles. The D - (-) - tartaric acid called, not entirely correct unnatural tartaric acid, is found only in the leaves of the West African orchid tree Bauhinia reticulata . The meso form does not exist in nature.
Manufacturing
The production of tartaric acid from tartar succeeds after the conversion into calcium tartrate. Tartaric acid can be released from this with sulfuric acid, gypsum is formed as a by-product . The meso form can be produced by oxidizing fumaric acid or maleic anhydride with hydrogen peroxide , potassium permanganate or other peracids .
Pure D - (-) - tartaric acid can be obtained from the racemate by breaking it down with brush mold Penicillium glaucum , since Penicillium glaucum only breaks down L - (+) - tartaric acid.
properties
The two carbon atoms that carry the two hydroxyl groups in the molecule of tartaric acid are stereocenters . Depending on the configuration of these centers, D - (-) - tartaric acid [synonym: ( 2S , 3S ) -tartaric acid], L - (+) - tartaric acid [synonym: ( 2R , 3R ) -tartaric acid] or the optically inactive meso - Tartaric acid. In the meso form, one of the stereocenters is ( R ) - the other ( S ) -configured . The right-handed L - (+) - shape occurs mostly in nature . The two enantiomers of tartaric acid [ L - (+) - tartaric acid and D - (-) - tartaric acid] do not differ in their physical and chemical properties only in terms of the rotation value against linearly polarized light. The rotation value α for L - (+) - tartaric acid + 12.7 °, with D - (-) - tartaric acid −12.7 ° under the same measuring conditions. As with all meso compounds, the rotation value of meso -tartaric acid is ± 0 °. The physiological properties of all three stereoisomers of tartaric acid are different.
| Isomers of tartaric acid | ||||
| Surname | D - (-) - tartaric acid | L - (+) - tartaric acid | meso -tartaric acid | |
| other names | ( 2S , 3S ) -tartaric acid | ( 2R , 3R ) -tartaric acid | ||
| Structural formula |

|
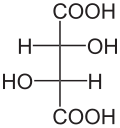
|
 Dashed line: mirror plane |
|
| CAS number | 147-71-7 | 87-69-4 | 147-73-9 | |
| 133-37-9 [ DL - (±) mixture] | ||||
| 526-83-0 (unspec.) | ||||
| EC number | 205-695-6 | 201-766-0 | 205-696-1 | |
| 205-105-7 [ DL - (±) mixture] | ||||
| 610-885-0 (unspec.) | ||||
| ECHA info card | 100.005.178 | 100.001.606 | 100.005.179 | |
| 100.004.642 [ DL - (±) mixture] | ||||
| 100.121.903 (unspec.) | ||||
| PubChem | 439655 | 444305 | 440015 | |
| 875 (unspec.) | ||||
| Wikidata | Q23034947 | Q18226455 | Q12447642 | |
| Q4111665 [ DL - (±) mixture] | ||||
| Q411237 (unspec.) | ||||
The alkali salts of tartaric acid are able to complex ( bind ) copper (II) ions in an alkaline solution and thus keep them in solution ( Fehling's solution ). Hydrogen tartrates can also be formed as a dibasic, relatively strong acid. A mixture of equal amounts of L - (+) - and D - (-) - tartaric acid ( racemate ) is called grape acid , melting point 205-206 ° C. This mixture is sometimes called racemic tartaric acid. A mixture of the three stereoisomers of tartaric acid with variable proportions L - (+) -, D - (-) - and meso -tartaric acid is traded as a mixture of isomers of tartaric acid.
use
Only L -tartaric acid is used on a larger scale , as it is the product of most synthesis processes for tartaric acid. 50% of the L (+) - tartaric acid produced is used in the food industry and pharmaceuticals, the other half in technical fields of application.
Tartaric acid is used as an ingredient in disinfectants. As a rule, it is not indicated whether it is L - or D - tartaric acid, the racemically mixed tartaric acid or another mixing ratio.
Use as a food additive
The most obvious application of tartaric acid is in its use as a food additive. L -tartaric acid, designated as E 334 in this area , is not only found naturally in many foods, but is also added to many mixed food products due to its taste and preservative properties. Tartaric acid is used in the preparation of ice cream, artificial honey , fruit, lemonades and soft drinks, jelly , wine gums and confectionery, e.g. B. to stabilize creams and foams, and used to acidify low-acid wines. The term tartaric acid is also incorrectly used in baking books. The oral toxicity of L -tartaric acid was extremely low in animal experiments with rats; the oral LD Lo for rats was 7500 mg / kg body weight.
Metatartaric acid (E 353), a polymer as a lactone , is mainly used to stabilize tartaric acid; as a colloidal protection, it prevents the crystallization of tartar from the wine.
Technical uses
Tartaric acid is also used in many technical areas, including making silk feel better and smooth . The ability of tartaric acid to form complexes with metals is significant : In these complexes, the metal cation is bound more tightly by the tartaric acid than in most other organic acids . This results in numerous possible uses. Potassium sodium tartrate is used, for example, as a complexing agent in Fehling's solution , while tartaric acid is used to treat the surface of copper and brass items. The latter can also be used to clean floors contaminated with heavy metals, as it binds toxic heavy metals here , but is itself biodegradable. If it is added to cement and plaster of paris , it delays their setting by complexing the calcium ions and thus extends the processing and deformability time. It also serves as a reducing agent and for the resolution of organic bases. In modern organic synthesis, LiAlH 4 -tartaric acid derivatives such as TADDOL are important chiral reagents or catalysts for the enantioselective reduction of ketones and other stereoselective synthesis processes.
Web links
Individual evidence
- ^ Alfred Henry Allen: Commercial Organic Analysis. Vol. I, 2nd Edition, J. & A. Churchill, 1885, p. 435, online at babel.hathitrust.org, accessed November 13, 2017.
- ^ A b c d Rudolf Wagner : Die Chemie. 6th edition, Wiegand, 1873, p. 515.
- ↑ Hans Meyer: Textbook of organic chemical methodology. Second volume, Springer, 1933, ISBN 978-3-662-37141-1 (reprint), p. 161.
- ↑ Entry on E 334: Tartaric acid (L (+) -) in the European database on food additives, accessed on June 27, 2020.
- ↑ a b Data sheet D - (-) - tartaric acid (PDF) from Carl Roth , accessed on August 7, 2010.
- ^ A b c K. Peter C. Vollhardt: Organic Chemistry. 1st corrected reprint of the 1st edition, VCH, Weinheim 1990, ISBN 3-527-26912-6 , p. 166.
- ↑ a b c The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition, 2006, ISBN 978-0-911910-00-1 , pp. 1557-1558.
- ↑ a b c d e Entry on tartaric acid. In: Römpp Online . Georg Thieme Verlag, accessed on November 12, 2014.
- ↑ a b Entry on tartaric acid in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b Entry on L - (+) - tartaric acid in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 31, 2018 or earlier.
- ^ Johann Christian Poggendorff : Biographical-literary concise dictionary. Volume 1: A – L , Barth, 1863, p. 1251.
- ↑ a b H. Hart, LE Crane, DJ Hart: Organic chemistry. 2nd edition, WILEY-VCH Verlag, Weinheim 2002, ISBN 978-3-527-30379-3 , p. 193.
- ↑ E. Breitmaier, G. Jung: Organic chemistry. 4th edition, Thieme Verlag, 2001, ISBN 978-3-13-541504-8 .
- ↑ S. Görtges: Metatartaric acid for tartaric stabilization ( Memento from August 15, 2016 in the Internet Archive ) (PDF). In: Switzerland. Z. fruit growing. No. 1, 2002, pp. 8-9.
- ↑ D. Seebach , AK Beck and A. Heckel: TADDOLs, Their Derivatives, and TADDOL Analogues: Versatile Chiral Auxiliaries. In: Angewandte Chemie International Edition . 40, 2001, pp. 92-138, PMID 11169693 .
- ↑ M. Aoki and D. Seebach : Preparation of TADOOH, a Hydroperoxide from TADDOL, and Use in Highly Enantioface- and Enantiomer-Differentiating Oxidations. In: Helvetica Chimica Acta . 84, 2001, pp. 187-207, doi : 10.1002 / 1522-2675 (20010131) 84: 1 <187 :: AID-HLCA187> 3.0.CO; 2-O .

