Organic acids
Organic acids are chemical compounds that have one or more functional groups or other structural elements thatenter into equilibrium reactionswith water or other solvents that can be protonatedby releasing protons. This creates the respective anions of the organic acids in question and the protons areabsorbedby the solvent water, whichactsas a proton acceptor ( base ), with the formation of oxonium ions H 3 O + . The concentration of the oxonium ions in the solution is then greater than the concentration of the hydroxide ions , so that the solutionbecomes acidic :
In addition to the organic acids, which already react with water as acids, there are a large number of other organic compounds that do not act against the weak proton acceptor water, but against stronger proton acceptors - e.g. B. the hydride anion H - or the amide anion NH 2 - can react as acids. In both cases, there is no equilibrium, but rather the formation of gaseous hydrogen or ammonia which escapes:
Such reactions are also acid-base reactions , but must be carried out in the absence of water and in a solvent that cannot itself donate protons (e.g. in diethyl ether ). Otherwise the very strongly basic anions hydride or amide would be protonated by the water or the unsuitable solvent and thus destroyed.
Grouping of organic acids
Preliminary remarks, acid strength
A measure of the acidity of a chemical compound is the acid constant or its pK s value . The value of the acid constant indicates how strongly the compound reacts ( protolyzes ) with water as an acid in the above equilibrium reaction , i.e. H. how far the above equilibrium is shifted to the right. The pK smaller: where s value, the greater is the acid strength of the organic acid.
The term organic acid is often rashly and simply equated with carboxylic acid , i.e. with compounds that carry one or more carboxy groups (-COOH). However, there are very many organic compounds that can react as acids, but which contain no carboxy group at all, but other groups that also give off a proton more or less easily, i. H. can react sourly. These can be groups such as B. the hydroxyl group in alcohols R-OH, or the sulfanyl group -SH in ( thiols ), or the group -SO 3 H in organic ( sulfonic acids ).
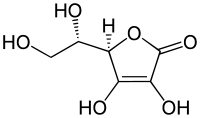
The release of a proton is all the easier, i. H. the more strongly the resulting anion is stabilized by mesomerism or other additional effects, the stronger the compound is . The compound ascorbic acid ( vitamin C ) is a particularly drastic example of this. There is no carboxy group in the molecular structure and yet this compound with p K S 4.25 is a stronger acid than acetic acid with p K S 4.75 because the anion of ascorbic acid is stabilized because of the enol groups present and possible tautomeric effects.
It is important to note that protons bound to C or N atoms can also be released if the anions formed are mesomeric stabilized. Such compounds are also referred to as CH-acidic or NH-acidic compounds. In the case of these compounds, in the absence of water as a solvent, the complete release of the proton can be forced by adding the strong reducing agent sodium , the protons being reduced to elemental hydrogen and thus removed from the equilibrium. Chemical syntheses can then be carried out with the anions of the CH or NH acidic compound produced in this way.
Carboxylic acids
Carboxylic acids with p K S values from 3.8 (formic acid) and higher are significantly stronger acids than alcohols with p K S values from 15.9 (ethanol) and higher. The acid strength of carboxylic acids increases even further if further electron-withdrawing groups are present near the carboxy group (in the α, β or γ position). So is z. B. trifluoroacetic acid with p K S 0.23 can be described as a strong acid, compared to unsubstituted weak acetic acid with p K S 4.75. Both acids are stronger acids than the 2-hydroxypropionic acid lactic acid with p K S 3.9, which in turn is only a slightly stronger acid than normal propionic acid with p K S 4.1.
Alcohols, phenols, enols
Un substituted alcohols (pK s greater than 16) have only very low acidities, while the phenols and naphthols because of mesomerism stabilization of the resulting phenates are clearly acidic phenol is a pK s value of 9.9 to a factor of 10 6 more acidic as z. B. ethanol (having a pK s of 16). Substituents on the aromatic ring systems of the phenols that have an -I- or -M-effect can greatly increase the acidity of phenols and naphthols by stabilizing the negative charge of the anions formed. If several such substituents are present, this can lead to strongly acidic compounds, such as. B. with picric acid (pK s 0.3) with three nitro groups (-M effect) on the aromatic ring, or with pentachlorophenol (pK s 4.7) (-I effect). In the specific case of enediol ascorbic acid , which the pK s is more acidic than 4.2 acetic acid (pK s 4.8) has already been made.
Thiols
Thiols have a higher acidity than the corresponding alcohols; the resulting thiolates are more stable than the alcoholates . This is shown by the comparison of the analogous compounds ethanol (pK s 16) and ethanethiol (also ethyl mercaptan , pK s 12).
Sulfuric acid esters and sulfonic acids
The Monoester the sulfuric acid and the sulfonic acids have a pK s value, which is comparable due to the similar structure with the pK s value of the second dissociation stage of sulfuric acid (pK s value of 1.9). The p-toluenesulfonic acid as having a pK s of 0.7. The mono-ester of sulfuric acid with lauryl alcohol , the so-called lauryl sulfate , is more acidic than sulfonic acid, has a pK s value of −0.09 and is therefore significantly less acidic than the proton of the first dissociation stage of sulfuric acid (pK s value −3).
Phosphoric acid esters and phosphonic acids
The mono- and diesters of phosphoric acid ( phosphoric acid esters ) and the organic derivatives of phosphonic acid (commonly called phosphonates ) are medium to weak acids, depending on the dissociation stage.
CH- and NH-acidic compounds
Even hydrocarbons without a carboxy, hydroxyl or sulfanyl group can act as acids due to their CH or NH acidity. The occurrence of multiple bonds and neighboring functional groups or heteroatoms is the cause of the acidity. The most famous CH-acidic compound is ethyne ( acetylene ), which due to the triple bond a pK s has value of 25 (ethane: 50). Further examples are nitroethane (pK s 8.6) and nitrocyclopentadiene , which is more acidic (pK s 3.3) than formic acid (3.75). During the deprotonation, a derivative of the aromatic cyclopentadienyl anion is formed from the nitrocyclopentadiene . Here, too, mesomeric effects together with inductive effects determine the acidity of the compounds. Acetonitrile is another example of a simple CH-acidic compound. Known NH-acidic compounds are phthalimide (pK s = 8.3), and sulfonylureas ( tolbutamide has a pKa of 5.16).
Overview
Substance class acidic functional group example pK s value at 25 ° C Carboxylic acids Carboxy group : R-COOH  acetic acid
acetic acid
4.8 Substituted
carboxylic acidsCarboxy group: R-CH x X y -COOH  Trifluoroacetic acid
Trifluoroacetic acid
0.23 aromatic
carboxylic acidsCarboxy group: Ar-COOH  Benzoic acid
Benzoic acid
4.2 Alcohols Hydroxy group : R-OH 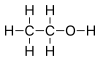 Ethanol
Ethanol
16 Phenols Hydroxy group: Ar – OH
(Ar = aryl )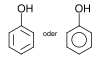 phenol
phenol
9.9 Naphthols Hydroxy group: Ar – OH
(Ar = aryl )α-naphthol 9.3 Substituted phenols
(substituents with -I effect
such as halogens , nitro groups )Hydroxy group: O 2 N – Ar – OH
(Ar = aryl ) Picric acid
Picric acid
0.4 Enols Hydroxy group (enol): C = C-OH  Ascorbic acid
Ascorbic acid
4.2 Thiols R-SH  Ethanethiol (C 2 H 5 -SH)
Ethanethiol (C 2 H 5 -SH)12 Sulfuric acid ester
( sulfate )R-O-SO 3 H Sulfuric acid dodecyl ester −0.09
value for the aciditySulfonic acids
(alkyl sulfonic acids)R-SO 3 H H 3 C-SO 3 H methanesulfonic acid −0.6 Sulfonic acids
(arenesulfonic acids)R – Ar – SO 3 H
(Ar = aryl )H 3 C-C 6 H 4 -SO 3 H p -toluenesulfonic acid ≈ 0.7 Phosphates
(= phosphoric acid ester )R-O-PO (OH) 2 Adenosine monophosphate 3.3 org. Phosphonic acids
(see phosphonates )R-PO (OH) 2 H 3 C-PO (OH) 2 methylphosphonic acid 2.35 CH-acidic compounds
alkynesH-C C-R 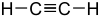 Ethine
Ethine
25th CH-acidic compounds
nitroalkanesO 2 N-CRR'-H 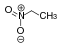 Nitroethane
Nitroethane
8.6 CH-acidic compounds
β-dicarbonylsRC = OCH 2 C = OR '  Acetylacetone
Acetylacetone
≈ 9 NH-acidic compounds
imidesR- (CO) 2 N-H  Phthalimide
Phthalimide
8.3 Inorganic acids for comparison Mineral acids HO-SO 2 -OH  sulfuric acid
sulfuric acid
−3
Individual evidence
- ↑ Alfons Hädener, Heinz Kaufmann: Fundamentals of organic chemistry. Springer, 2006, ISBN 978-3-7643-7040-4
- ^ A b Hans Beyer and Wolfgang Walter : Organische Chemie , S. Hirzel Verlag, Stuttgart, 1984, p. 521, ISBN 3-7776-0406-2 .
- ↑ Entry on tolbutamide in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 31, 2018 or earlier.
- ↑ a b c d chem.wisc.edu: pKa Data , Compiled by R. Williams (PDF, 78 kB).
- ↑ a b CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Rappoport, Zvi., Frankel, Max .: Handbook of tables for organic compound identification . 3d ed. Chemical Rubber Co, Cleveland 1967, ISBN 0-8493-0303-6 .
- ↑ ChemieOnline letter E (ethyl mercaptan) .
- ^ PH Stahl, CG Wermuth: Handbook of Pharmaceutical Salts: Properties, Selection, and Use , 2002, Helvetica Chimica Acta, ISBN 3-906390-26-8 .
- ↑ ChemieOnline letter P
- ↑ ChemieOnline letter N
- ↑ ChemieOnline letter A