Pentachlorophenol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
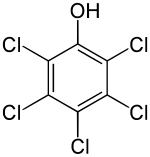
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Pentachlorophenol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 HCl 5 O | ||||||||||||||||||
| Brief description |
white, odorless, needle-shaped crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 266.35 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.98 g cm −3 |
||||||||||||||||||
| Melting point |
190-191 ° C |
||||||||||||||||||
| boiling point |
decomposition |
||||||||||||||||||
| Vapor pressure |
8 m Pa (20 ° C) |
||||||||||||||||||
| pK s value |
4.7 (25 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Pentachlorophenol ( PCP for short ) is a chlorinated, aromatic hydrocarbon and is a phenol derivative in which all aromatic hydrogen atoms have been replaced by chlorine . In 2015 it was included in Appendix A of the Stockholm Convention .
presentation
Pentachlorophenol can be obtained by chlorination of phenol, or by hydrolysis of hexachlorobenzene be prepared. The production of PCP was severely restricted due to its ecotoxicological properties.
properties
Pentachlorophenol is colorless, solid at ambient temperature and very soluble in fat. Technical PCP and its sodium salt usually contain highly toxic impurities such as polychlorinated dibenzodioxins and furans (PCDD or PCDF). Dioxins and furans can also be formed when products containing PCP are incinerated or when exposed to light. PCP has bactericidal and fungicidal properties and is therefore suitable for numerous areas of application. Pentachlorophenol does not occur naturally in the environment; it is only released by humans.
Pentachlorophenol is subject to intensive photomineralization and is also biologically metabolized to pentachloroanisole and p - dimethoxytetrachlorobenzene . These products are, relative to pentachlorophenol, photochemically stable metabolites and can be detected in soils, sediments and plants. The metabolism of pentachlorophenol in aerobic aquatic natural mixed cultures results in pentachloranisole as the main product. Its photostability is similar to that of hexachlorobenzene , which due to its physicochemical properties causes ubiquitous distribution through the atmosphere. This leads to an accumulation in lipophilic parts of the environment, e.g. B. in conifer growth or in fish fat. The combustion of the pure substance pentachloroanisole gives hexachlorobenzene as the main product and thus a tertiary product of PCP.
use
Because of its fungicidal effect, PCP was mainly used in wood preservatives , for example in West Germany in the wood preservative Xylamon BV (in combination with lindane ), in the GDR in the agents Hylotox IP and Paratectol 9025, among others . In some countries, PCP, however, still in the textile and leather industry as well as impregnating and blue stain for wood used. It is to be proven u. a. in textiles made from natural fibers and printing inks.
Biological effect
PCP decouples the respiratory chain in the mitochondria of the cells, which leads to an increase in blood pressure, hyperglycemia , accelerated breathing and heart failure. It is absorbed both through the gastrointestinal tract and through the skin . The biological half-life of PCP is around 1 to 3 weeks. It should be noted that, due to the manufacturing process, PCP is mostly contaminated with dioxins , the toxicity of which exceeds that of PCP, depending on the degree of contamination.
Today, wood treated with PCP is a serious building pollutant in many existing buildings , which often requires extensive renovation.
safety instructions
Wood treated with PCP has particularly high levels of the compound in the top layers, where values in the order of several 1,000 mg / kg are measured. Because of the long retention time in the wood, higher values are found than in untreated wood many years after use. But even with untreated wood, the values can be increased if the raw wood has been treated or secondary contamination occurs. PCP gradually evaporates from treated wood and attaches to other surfaces, e.g. B. on house dust, wallpaper, plaster, furniture, textiles or books. PCP also accumulates in clothing materials such as wool, linen and cotton. It should also be noted that PCP can penetrate rubber gloves in oily solutions. The low water solubility of 14 mg / l (at 25 ° C) and the low biodegradability lead to a ubiquitous occurrence.
In order to determine the indoor exposure to the wood preservative PCP , the time, type and amount of use must first be determined. If the quotient of the treated wood surface and the room volume is over 0.2 m² / m³, a dust analysis of fresh dust (around a week old) or old dust that has been deposited for a longer period must be carried out. At concentrations of more than 1 mg PCP / kg fresh dust or more than 5 mg PCP / kg old dust, material samples are taken from 0 to 2 mm depth of the wood. If this results in a value of over 50 mg PCP / kg wood, the annual mean indoor air pollution must be determined. According to the PCP directive, remediation is required if the annual mean concentration is more than 1 μg PCP / m 3 air.
Since 1985 the industry association for construction, chemistry and wood preservation has made a voluntary commitment not to use PCP in its products. The production of pentachlorophenol in the Federal Republic of Germany has been suspended since 1986. In 1989 the federal government issued the Pentachlorophenol Prohibition Ordinance on the basis of the Chemicals Act. Since then, the manufacture, placing on the market and use of PCP, Na-PCP and products containing PCP that contain more than 5 mg / kg PCP has been prohibited. In addition, the total input of dangerous polychlorinated dibenzodioxins and dibenzofurans (PCDD and PCDF), which are also contained as impurities in technical PCP, is significantly reduced. In 1996, the contents of the PCP regulation were incorporated into the chemicals prohibition regulation that has been in effect since then (Section 15). Since the PIC Convention came into force in 2004, PCPs have only been allowed to be exported to developing countries if they have been informed of the risk potential and have expressly agreed.
proof
Pentachlorophenol can be detected by color reactions, more precise determinations can be made by UV spectroscopy or thin layer chromatography . A gas chromatographic analysis is usually carried out as methyl, ethyl ether or in acetylated form. The determination of PCP in wood after acetylation by means of GC-ECD ( electron capture detector ) is described in Appendix IV (to § 6) of the Waste Wood Ordinance , the determination in leather in DIN EN ISO 17070. Instead of the electron capture detector (ECD), a mass-selective detector ( Mass spectrometer , MS) can be used.
UV spectroscopy is used to detect the bathochromic and hypochromic shift in absorption, which occurs when PCP interacts with a cyclodextrin - porphyrin complex ("capped cyclodextrin"). The formation of hydrogen bonds between the hydroxyl group of the phenol and the nitrogen of the pyrrole leads to a change in the porphyrin absorption.
Web links
- Substance monograph pentachlorophenol - reference and human biomonitoring values , Bundesgesundheitsbl - Gesundheitsforsch - Gesundheitsschutz , 1997, 40, No. 6, doi : 10.1007 / BF03042913 .
- Heidelore Fiedler, Matthias Hilpert, Michael Hub, Otto Hutzinger : Handbook of Contaminated Sites and Groundwater Damage Cases - Report on Pentachlorophenol (PCP) (PDF; 2.4 MB), State Institute for Environmental Protection. B. den-Württemberg, Karlsruhe 1996.
- Biological degradation pathways of pentachlorophenol
- Pentachlorophenol (PCP) detailed information and guide values (UmweltWissen - Bavarian State Office for the Environment; PDF; 309 kB)
Individual evidence
- ↑ a b Entry on pentachlorophenol. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ↑ a b c d e f g Entry on pentachlorophenol in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b Entry on pentachlorophenol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Entry on pentachlorophenol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 87-86-5 ), accessed on March 4, 2020.
- ↑ Countries move forward on important issues for sustainable management of chemicals and waste: “[…] four new listings (three under the Stockholm and one under the Rotterdam Conventions - polychlorinated napthalenes, hexachlorobutadiene, and pentachlorophenol and its salts and esters; and methamidophos respectively ) ” , 2015.
- ↑ a b Bavarian State Office for the Environment: Pentachlorophenol (PCP)
- ↑ KW Schramm, A. Reischl, Monika Hirsch, D. Lenoir, O. Hutzinger: Pentachlorophenol - Secondary and tertiary conversion products In: Environmental sciences and pollutant research. 1, 1989, p. 6, doi : 10.1007 / BF02983900 .
- ↑ TGL 4424, online at holzfragen.de: The compatibility of the active ingredients in various wood preservatives - GDR wood preservatives , accessed on April 6, 2012
- ↑ a b allum.de: Pentachlorphenol , accessed on March 30, 2013.
- ↑ Bavarian State Office for Health and Food Safety: Pentachlorophenol (PCP)
- ↑ Institute for Occupational Safety and Health of the German Statutory Accident Insurance (IFA): Indoor workplaces - Recommended procedure for investigating the work environment. Retrieved March 11, 2020 .
- ^ U. Schlottmann: PCP Prohibition Ordinance entered into force. In: Environmental sciences and pollutant research. 2, 1990, p. 41, doi : 10.1007 / BF03039332 .
- ↑ Rotterdam Convention: Chemicals listed in Annex III of the Convention and currently subject to the PIC Procedure
- ↑ Der Tagesspiegel : Poison exports to developing countries are restricted
- ↑ Shishan Zhao, John HT Luong: A cyclodextrin-porphyrin assembly as chemosensor for pentachlorophenol . In: Journal of the Chemical Society, Chemical Communications . No. 6 , 1995, pp. 663-664 , doi : 10.1039 / C39950000663 .


