Hydrogen bond
| Hydrogen bond |
|
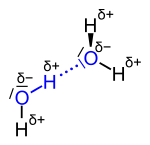
| The hydrogen bond between two water molecules; the atoms involved are shown in blue , the actual bridge bond with blue dots . |
Hydrogen bond , also short hydrogen bond or H- bond , is an attractive interaction of a covalently bonded hydrogen atom (R 1 −X − H) usually with a free electron pair of an atom Y of an atom grouping | Y − R 2 . This interaction only occurs when X is more electronegative than hydrogen, i.e. when H is polar bound. The interaction of the hydrogen atom with Y is enhanced when | Y is electronegative. Hydrogen bonds are often shown in the form R 1 −X − H … | Y − R 2 as a dotted line. As electronegative atoms, nitrogen (N), oxygen (O) and fluorine (F) are of particular importance because they have the highest electronegativity values (EN).
Hydrogen bonding is a form of minor valence bond and its strength is usually well below that of a covalent bond and ionic bonds . Hydrogen bonds between molecules lead to a high melting and boiling point of the compound in relation to the molar mass. Interactions within and between molecules determine the structure of peptides and nucleic acids .
discovery
The concept of hydrogen bonds was first described in 1919 by Maurice L. Huggins and in 1920 by Wendell Mitchell Latimer and Worth H. Rodebush to explain the high dielectric constant of water.
Structure of the bond

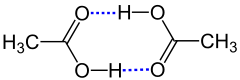
Hydrogen bonds exist when two functional groups interact via hydrogen atoms . It does not matter whether the bond is between two molecules or two distant sections of a - mostly large - molecule. A distinction is made between the functional groups between the proton donor (also: donor , donor ) and the proton acceptor . The donor is an electronegative atom (e.g. nitrogen , oxygen or fluorine ) to which a hydrogen atom is covalently bonded. The acceptor is any other atom with lone pairs of electrons . The bond between the hydrogen atom and the acceptor is usually weaker (longer) and is symbolized as a dotted line. In general, such a system is represented as follows:
- .
Certain functional groups can act as donors and acceptors at the same time. A simple example is the hydrogen bonds between water molecules. Here X and Y are oxygen atoms:
- .
The electronegativity difference of the covalently bonded atoms and the hydrogen bond itself create partial charges . A positive partial charge (δ + ) sits on the hydrogen atom of the hydrogen bridge bond and negative partial charges (δ - ) sit on the donor X and the acceptor Y.
The atoms X − H … | Y are often arranged linearly (bond angle close to 180 °) and the bond can be viewed as a 2-electron-3-center bond . The bond is not only electrostatic ( ionic ), even the weak H … | Y bond part has a direction of action, similar to covalent bonds. The alignment of the lone pairs of electrons of the acceptor Y directs the angle between H … | Y – R 2 , which is therefore mostly not linear.
A hydrogen bond can be viewed as a “frozen” partial step in a proton transfer . The strength of the bond increases with the acid constant of R − X − H and the base constant of | Y − R. In the case of a hydrogen bond between an oxonium ion (H 3 O + ) and a water molecule, a dimer H (OH 2 ) 2 + with a strong bond is formed, in which the two bonds of the bridging H atom are of equal length:
- with 138 kJ / mol.
If there are hydrogen bonds between binding partners that are difficult to polarize and have weak acid-base behavior, the hydrogen bonds are very weak and undirected. In this case, the strength of the interaction is determined by van der Waals forces .
Classification of hydrogen bonds
George A. Jeffrey introduced a classification based on the strength of hydrogen bonds.
- Strong bonds (63–167 kJ / mol): For example, the hydrogen bond of hydrogen fluoride
- Medium bonds (17–63 kJ / mol): For example, the hydrogen bonds in water or in carbohydrates
- Weak bonds (<17 kJ / mol): For example, C − H ··· O hydrogen bonds in proteins
Effects of hydrogen bonds
Hydrogen bonds as intermolecular forces act between molecules of a compound or the compound with protic solvents such as water. You lead to
- the miscibility of short-chain alcohols with water and
- the good solubility of gases such as ammonia or carbon dioxide in water,
- the water solubility of simple saccharides ( monosaccharides , disaccharides ) and polymers such as polyethylene glycol ,
- increased boiling and melting points as well as increased enthalpies of vaporization of many compounds that carry hydroxyl or amino groups .


Comparison of the boiling points and molar mass M of alkanols and alkanes : Compared to alkanes of similar mass, alkanols have a higher boiling point. Comparison of the boiling points and molar masses of hydrogen compounds of the 4th main group and the 6th main group of the elements: Water has a noticeably high boiling point. In the case of hydrogen sulfide (H 2 S), the hydrogen bonds are already very weak and have hardly any effect on the boiling point.
Hydrogen bonds in biomolecules
Hydrogen bonds are responsible for the special properties of many molecules that are important for living things :
- Proteins : Stabilization of secondary structural elements such as α-helix and β-sheet , as well as the tertiary structure and quaternary structure (other types of binding also occur with proteins).
- RNA : complementary base pairing within ncRNA molecules or between RNA and DNA molecules.
- DNA : complementary base pairing within the double helix ; the two strands of DNA are held together by the hydrogen bonds. However, they can be detached (when copying with helicopters ) (“zip” principle).
- Active ingredients : The binding affinity of active ingredients to their target structures depends largely on the hydrogen bonds formed.



Representation of a β-sheet. R stands for the remainder of the respective amino acid, hydrogen bonds are shown in dashed lines. The base pair GC { guanine (G) and cytosine (C)} contains three dotted blue hydrogen bonds. The base pair AT { adenine (A) and thymine (T)} contains two dotted blue hydrogen bonds.
Hydrogen bonds of water
Due to the higher electronegativity of oxygen with 3.4 compared to that of hydrogen with 2.2, the water molecule has partial charges . The oxygen is partially negative (δ−), the hydrogen atoms partially positive (δ +). The hydrogen bonds form between the different partial charges.
Hydrogen bonds are responsible for a number of important properties in water . These include the liquid state of aggregation under normal conditions , the cohesion , the relatively high boiling point and the density anomaly of the water .
The typical bond length of hydrogen bonds in water is 0.18 nm. There are two types of bonds. So-called linear bonds with a bond angle of 180 ° and non-linear 180 ° ± 20 °, whereby the linear bond predominates. Whereas a purely tetrahedral network (bond angle 180 °) would have to lead to 4 closest neighbors (coordination number 4), the coordination number measured (by X-ray scattering ) is 4.5 under normal conditions. With decreasing density, this measure of order decreases (in contrast to an increase in the coordination number for most other liquids) to 4 and thus to the value for an ideal tetrahedral structure.
The hydrogen bonds must be broken during evaporation ; This explains the high energy expenditure (compared to other substances) to convert liquid water at 100 ° C into steam at 100 ° C (see heat of vaporization ).
Intramolecular hydrogen bonds
If there are several donors / acceptors in a molecule, hydrogen bonds can form within the molecule, as is the case with ricinoleic acid . Both a hydroxyl group and a carboxy group are present there. Actually, the hydroxyl group should increase the melting or boiling point. However, the boiling point of ricinoleic acid is even lower than that of oleic acid , which only differs in the lack of a hydroxyl group, since the hydroxyl group forms a hydrogen bond with the carboxy group. So the carboxy group can not to the same extent more inter enter into molecular hydrogen bonds than if the intra were not present molecular hydroxy group. The spatial structure is also changing; pseudocyclic structures arise due to the forces of attraction between the polar groups.
literature
General textbooks
Special books
- George A. Jeffrey: An Introduction to Hydrogen Bonding . Oxford University Press, 1997, ISBN 978-0-19-509549-4 .
- George C. Pimentel, AL McClellan: Hydrogen Bond . WH Freeman & Co Ltd., San Francisco 1960, ISBN 978-0-7167-0113-2 .
- Anthony J. Stone, AJ Stone: The Theory of Intermolecular Forces . Oxford University Press, Oxford 1997, ISBN 978-0-19-855883-5 .
Web links
Individual evidence
- ↑ Huggins, 50 Years Theory of Hydrogen Bonding, Angewandte Chemie, Volume 83, 1971, pp. 163-168. He claims to have been the first to introduce the concept, in his thesis in advanced inorganic chemistry at Berkeley in 1919. As support, he mentions his teacher Gilbert Newton Lewis , Valence and the Structure of Atoms and Molecules , New York, 1923, p. 109, who confirms this ( The idea was first suggested by Dr. ML Huggins and was also advanced by Latimer and Rodebush ). However, Huggins did not publish about it until 1922 (Science, Volume 55, 1922, p. 459, Phys. Rev., Volume 19, 1922, p. 346, J. Phys. Chem., Volume 26, 1922, p. 601). Latimer and Rodebush themselves refer to Huggins in their original work (J. Am. Chem. Soc., Volume 42, 1920, p. 1419). Huggins, Latimer, and Rodebush were colleagues at Berkeley.
- ↑ Wendell M. Latimer, Worth H. Rodebush: Polarity and Ionization from the Standpoint of the Lewis Theory of Valence . In: Journal of the American Chemical Society . tape 42 , 1920, pp. 1419–1433 , doi : 10.1021 / ja01452a015 ( full text ).
- ↑ George A. Jeffrey: An Introduction to Hydrogen Bonding . Oxford University Press, 1997, ISBN 978-0-19-509549-4 .
- ↑ Lin Jiang, Luhua Lai: CH ⋯ O Hydrogen Bonds at Protein-Protein Interfaces . In: Journal of Biological Chemistry . tape 277 , no. 40 , 2002, p. 37732-37740 , doi : 10.1074 / jbc.M204514200 .
- ^ L. Pauling, RB Corey, HR Branson: The Structure of Proteins: Two Hydrogen-Bonded Helical Configurations of the Polypeptide Chain . In: Proceedings of the National Academy of Sciences . 1951, p. 205-211 .
- ^ L. Pauling, RB Corey: Configurations of polypeptide chains with favored orientations of the polypeptide around single bonds: Two pleated sheets . In: Proceedings of the National Academy of Sciences . tape 37 , 1951, pp. 729-740 .
- ^ MA Williams, JE Ladbury: Hydrogen Bonds in Protein-Ligand Complexes . In: H.-J. Böhm, G. Schneider (Ed.): Protein-Ligand Interactions . Wiley-VCH, Weinheim 2005, ISBN 978-3-527-30521-6 , pp. 137-161 , doi : 10.1002 / 3527601813.ch6 .